Abstract
Trauma and environmental stressors leave biological marks that can shape mental health across an individual’s lifespan. Through processes like DNA methylation, histone modification and non-coding RNAs, stress becomes “written” into gene regulation, influencing vulnerability to conditions such as post-traumatic stress disorder (PTSD) and depression. This paper reviews evidence across different stages of life: beginning with PTSD and the psychological impacts of trauma; secondly, examining prenatal exposures such as maternal depression; and finally, extending to broader environmental, psychological and physical stressors in adulthood. We also highlight new avenues for treatment, including psychedelics, which may restore neural plasticity, and artificial intelligence (AI), which is being used to detect risk, predict outcomes and guide just-in-time interventions. Taken together, these findings show how epigenetics offers a unifying lens for understanding the long reach of trauma, while AI and emerging therapies provide opportunities for more timely and personalised approaches to prevention and care.
Introduction
The effects of trauma and stress on mental health are far-reaching, influencing not only individuals who directly experience them but also, in some cases, future generations. Post-traumatic stress disorder (PTSD) is perhaps the most visible example, long recognised as a consequence of exposure to extreme events such as war. Depression, too, has become one of the most common global health concerns, worsened not only by direct trauma but also by ongoing social, physical and environmental pressures.
Epigenetic research provides a way of understanding how these experiences can become biologically embedded. Unlike genetic mutations, epigenetic changes do not alter the DNA sequence but instead regulate how genes are expressed. Key mechanisms include DNA methylation, histone modifications and non-coding RNAs, which can alter the activity of stress-related genes such as NR3C1, SLC6A4 and BDNF. These modifications help explain why stress can have lasting effects on behaviour, cognition and emotional regulation.
This review is organised around the human life course. We begin with prenatal and perinatal exposures, such as maternal depression and antidepressant use, and their effects on foetal development. We then turn to childhood and adolescence, where caregiving environments and early adversity can shape stress-response systems. Next, we examine adult stressors, from psychological challenges to physical and environmental pressures, and how they interact with epigenetic pathways. Finally, we discuss emerging interventions, including psychedelics, which can promote neuroplasticity, and artificial intelligence (AI) based tools, which are changing how risk is detected and care is delivered. By moving chronologically, we aim to show both how stress accumulates over time and how targeted interventions may interrupt this cycle.
I. PTSD and Trauma
Post-traumatic stress disorder (PTSD), has long been recognised as a severe consequence of exposure to traumatic events, impacting individuals across various demographics (American Psychiatric Association, 2013). Historical conflicts, such as World War I and II, brought widespread attention to the psychological toll of warfare, leading to early understandings of what was then termed “shell shock” or “combat fatigue” (Jones et al., 2007). These historical observations laid the foundational groundwork for understanding the lasting psychological scars of profound stress.
The pervasive impact of war extends beyond direct combatants to affect entire populations, leading to chronic stress, displacement and economic hardship that profoundly influence mental wellbeing (Mollica et al., 2019). The intergenerational transmission of trauma, where the effects of severe stress are observed in the offspring of those who experienced it, further highlights the long-reaching consequences of such events; for instance, studies of Holocaust survivors have shown that trauma-related changes can be passed down to subsequent generations (Yehuda & Bierer, 2009). These historical and ongoing stressors contribute to a complex landscape of mental health challenges that continue to evolve.
Presently, depression stands as a leading global mental health concern, affecting millions worldwide regardless of their direct exposure to acute trauma (World Health Organization, 2021). While not always linked to a single traumatic event, chronic environmental stressors, such as socioeconomic disparities, discrimination and climate change related anxieties, contribute significantly to its prevalence and severity. Understanding the interplay between these past and current stressors – and their epigenetic consequences – is crucial for developing effective prevention and intervention strategies in mental health.
II. Maternal Depression: Epigenetic Effects on the Child
Maternal depression during pregnancy has been linked to certain epigenetic changes in the foetus. These changes are often seen in genes that regulate stress responses, brain growth and mood-related chemical messengers. The effects can be observed in the umbilical cord’s blood, placenta and infant cells soon after birth; in some cases, they predict later stress reactivity or behavioural differences in the child. Importantly, these changes are not uniform; they can vary depending on the timing of maternal depression during pregnancy, the baby’s sex and postnatal caregiving (Conradt et al., 2013; Braithwaite et al., 2015; Viuff et al., 2018; McGill et al., 2022). During normal early development, the foetus undergoes extensive epigenetic reprogramming, a process where most parental DNA methylation marks are erased and then re-established to allow proper tissue differentiation and developmental programming (Reik et al., 2001; Messerschmidt et al., 2014). This creates a highly sensitive environment where the foetal epigenome can respond to internal and external cues, including maternal physiology (Monk et al., 2016). Under normal circumstances, this reprogramming ensures the correct activation of genes critical for growth, neurodevelopment and metabolic regulation (Feng et al., 2010).
EPIGENETIC CHANGES TO THE FOETUS
Maternal depression can cause an increase in DNA methylation on the glucocorticoid receptor gene – a gene important for controlling the stress hormone system – in the umbilical cord and cheek cells of the foetus (Hompes et al., 2013; Braithwaite et al., 2015). This may weaken the baby’s ability to regulate stress hormones, leading to stronger stress reactions later in life (Hompes et al., 2013; Braithwaite et al., 2015). Maternal stress hormones like cortisol, which are released in higher amounts in depression, are usually not able to freely cross the placental barrier (Conradt et al., 2013). However, depression can reduce the activity of the 11β-HSD2 enzyme that normally inactivates cortisol and prevents excess foetal exposure (Conradt et al., 2013; Appleton et al., 2014). Excess cortisol can alter foetal brain development by changing neuronal growth and connectivity, which increases vulnerability to emotional and cognitive difficulties later in life (O’Donnell et al., 2017). In some studies, placentas from female foetuses show a greater activity of protective enzymes like 11β-HSD2, while placentas from male foetuses can be less protected and more vulnerable (Clifton, 2010). Another gene that can be affected is the Brain-Derived Neurotrophic Factor (BDNF), which supports brain cell growth, connections and neuroplasticity (Bath & Lee, 2006). Maternal depression can increase DNA methylation in the BDNF promoter region, potentially lowering BDNF levels in the baby and affecting brain development and resilience to stress (Braithwaite et al., 2015). Furthermore, the serotonin transporter gene SLC6A4, which controls how serotonin is recycled in the brain, is also influenced (Heils et al., 1996). Higher DNA methylation of this gene is possible owing to the exposure of maternal depression on a foetus, which may alter serotonin function and contribute to later emotional difficulties (Devlin et al., 2010; Oberlander et al., 2008). Epigenetic clocks are measures that compare DNA methylation patterns to biological age; it has been noted that foetuses exposed to maternal depression may have a slightly slower epigenetic age at birth, which could reflect changes in their developmental pace (McGill et al., 2022). Epigenome-based studies, which study the DNA methylation patterns across the whole genome of the foetuses, indicate that maternal depression can lead to subtle changes that are widespread across the genome (Viuff et al., 2018).
The effect on the foetus is also very much dependent on the timing of exposure to maternal depression. Exposure can still affect foetal growth and stress pathways, but may not alter prime developmental processes as drastically in the late gestation period (Monk et al., 2019). Table 1 shows the detailed effects and implications of maternal depression in each trimester of pregnancy. In addition, the persistence of epigenetic changes in the germline can suggest that the transmission of epigenetic marks is majorly caused by an incomplete erasure of epigenetic marks during re-programming (Franklin et al., 2010).
| – | Effects of Maternal Depression | Implications | References |
| Early gestation (0-13 weeks) |
DNF disruption affecting neural tube and synapse formation | Cognitive delays due to disrupted neuroplasticity | Heijmans et al. (2008) |
| Mid gestation (14-27 weeks) |
Certain unfavourable epigenetic changes in immune genes | Altered immune function and higher risk of allergies/asthma | O’Donnell & Meaney (2017) |
| Late gestation (28-40 weeks) |
Cortisol/serotonin exposure alters brain connectivity | Higher risk of ADHD and emotional dysregulation | Devlin et al. (2010); O’Donnell et al (2017) |
Table 1: Effects and implications of maternal depression in each trimester.
EFECTS OF MATERNAL DEPRESSION ON COLOSTRUM
Colostrum (breast milk) is the ideal source of nutrition in infants and is superior to formula milk (American Academy of Pediatrics, 2022). Colostrum also contains microRNAs and other epigenetic regulators that can survive digestion and influence gene expression in the infant (Zhou et al., 2012). Alterations in these signals due to maternal depression can therefore contribute to epigenetic programming effects in the infant. For example, increased exposure to cortisol via breastmilk has been associated with differences in DNA methylation of the glucocorticoid receptor (GR) gene, which plays a key role in stress hormone regulation (Hinde et al., 2015; Conradt et al., 2016). Similarly, changes in immune-related microRNAs could affect the infant’s immune system development through postnatal epigenetic changes (Alsaweed et al., 2016).
EFFECTS ON THE FOETUS OF CONVENTIONAL DEPRESSION TREATMENT
Depression in adults is usually treated with antidepressant drugs, such as selective serotonin re-uptake inhibitors (SSRIs) and tri-cyclic antidepressants (Patel et al., 2023). However, they are prescribed with a strong caution during pregnancy. This can be seen in the significant drop of antidepressant use from 6.6% before pregnancy to 3.7% on the first day of gestation; similarly, 7.7% of antidepressant use was below the recommended daily dosage (Ramos et al., 2007).
These drugs can easily cross the placenta and can affect the foetus by direct exposure as their levels are measurable in the amniotic fluid and umbilical cord blood (Hendrick et al., 2003). This can lead to several issues, including neonatal adaptation syndrome (NAS), which can cause jitteriness, respiratory distress, hypoglycemia, sleep problems and irritability, and can occur in 20-30% of exposed neonates (Sanz et al., 2005; Levinson-Castiel et al., 2006; Hendson et al., 2021). Moreover, it is also commonly linked with persistent pulmonary hypertension of the newborn (PPHN). This occurs on its own very rarely, however infants exposed to SSRIs during the late stages of pregnancy have an increased risk of developing PPHN. Antidepressants can also alter foetal hypothalamus-pituitary-adrenal (HPA) axis activity, potentially changing cortisol reactivity later in the infant’s life (Oberlander et al., 2008).
It is important to understand that in clinical settings where risks and benefits are weighed proportionally, it can be a better option to take or to continue to take SSRIs during pregnancy, as untreated depression can lead to much more severe complications in both the mother and the child (American College of Obstetricians & Gynecologists, 2023). In general, this still remains a topic for more research, as it is currently difficult to separate the effects of maternal depression itself and the effects of antidepressant treatment on the foetus.
III. Environmental, Psychological and Physical Stressors
III.I ENVIRONMENTAL STRESSORS
The phenomenon of rapid evolution remains a subject of scientific uncertainty, yet it has been associated with the similarly complex and not fully understood processes of epigenetic modification. Epigenetic modification refers to heritable changes in gene function that do not involve alterations to the DNA sequence itself. These include DNA methylation, histone modifications, chromatin remodelling and regulation by non-coding RNAs; these changes can induce the phenotypic modifications that can be morphological, physiological or behavioural at the organismal level. For example, the athletic ability of an individual can be primarily compromised by their environment and their genetics.
While the genetic aspect of inheritance is widely understood and agreed upon, the influence of the environment continues to puzzle researchers to this day. However, recent discoveries have begun to clarify the mechanism behind this phenomenon. The same genes can have different patterns of expression in response to environmental cues. For example, NR3C1 (the glucocorticoid receptor gene) undergoes DNA methylation changes in response to early life stress, trauma or chronic stress, which in turn affects cortisol sensitivity. Similarly, SLC6A4 (the serotonin transporter gene) becomes methylated as a result of stress and social environment, increasing the risk of depression and anxiety. These environmental exposures can guide DNA methylation or demethylation processes and may even instigate C > T mutations. Furthermore, epigenetic markers such as DNA methylation (DNAm) – the addition of methyl groups to DNA that can regulate gene activity – can serve as biomarkers of these epigenetic modifications, further contributing to variation in an organism’s phenotype. DNAm has also recently been linked to cardiovascular disease. Additionally, these stress-related epigenetic changes may serve as early indicators of risk for cardiometabolic abnormalities at specific genomic loci (Perera & Silvestre, 2023).
Research on asexual organisms has proven effective for studying how organisms respond to environmental stressors without the confounding influence of genetic variation. As these organisms have limited genetic diversity, researchers can observe multiple generations with nearly identical genetic codes. Epigenetics can also aid in understanding how bacteria, fungi and protoctists adapt to different environments. Although these invasive species have little genetic diversity, epigenetic variation allows them to thrive in environments where they might otherwise fail, often due to DNA methylation (DNAm) changes. Studies have shown that epigenetic variation plays a key role in enabling organisms to adapt to their surroundings. In particular, DNA methylation can regulate the expression of stress-related genes. For instance, asexual organisms are able to adjust to drastic changes in salinity or drought conditions through the activation of genes involved in osmotic stress responses, including those responsible for synthesising osmoprotectants such as proline. This allows the organism to adapt within a single generation, highlighting the impact of environment factors on all organisms.
III.II Psychological Stressors
The study of psychological disorders has a long history, and recent research highlights the substantial role of epigenetics in their development. Stressors have been known to interact with the genome to produce stable changes in DNA structure and expression, with these mechanisms possibly underlying the pathological behaviours observed in individuals (Jakobsson et al., 2008). Crucially, it is important to note that stress and depression have predominantly been associated with epigenetic alterations in genes involved in mediating resilience and/or vulnerability to stress, including stress-response related genes (e.g., NCR31CRF), genes involved in neurotransmission (e.g. SLC6A4) (Klengel et al., 2013; Tsankova, et al., 2006) and neurotrophin genes (e.g. BDNF, GDNF) (Ellicott, et al., 1990). For example, a preclinical study demonstrated that exposure to stress in rats has been associated with changes to the epigenome (Ben David et al., 2023), showing that chronic unpredictable stress altered histone acetylation in the rodents’ forebrains which increased anxiety-related behaviour and increased vulnerability to stress-induced cognitive impairments. Stress can be described as the consequence of physical or psychological trauma that induces physiological responses to cope with the event. A key mechanism involved in this process is the activation of the HPA axis. The HPA axis is a neuroendocrine system that mediates stress adaptation through metabolic and behavioural changes (O’Donnell et al., 2017). Negative feedback in gene regulation heightens the risk of psychiatric disorders such as mood disorders. This can be caused by stressful events which can negatively affect behaviour and promote the risk of these disorders. These stressors – often early-life, severe and/or chronic – have been associated with increased risk for the onset of major depression and bipolar mood symptoms in clinical studies (Ellicott et al., 1990). Ultimately, this is caused by altering the expression of genes involved in neurotransmission through changes to DNA methylation patterns. A well-studied example is the serotonin transporter gene SLC6A4 which has been linked to epigenetic modification repeatedly (Heils et al., 1996; Oberlander et al., 2008).
III.III Physical Stressors
There are a wide range of physical stressors which have been associated with epigenetic modifications, including nutrient deprivation, extreme weather and toxins/pollutants (Hing et al., 2014; Park et al., 2019). Among other factors, extreme weather has emerged as a significant driver of epigenetic modification in the context of climate change. For example, in the Hevea brasiliensis tree crop, cold and drought stress have been shown to cause changes in specific regions of the genome – known as differentially methylated regions (DMRs) – at cis-regulatory sites (Uthup et al., 2011).
Heat stress can also modify epigenetic patterns, including DNA methylation and histone modification, especially in plants and some animals. For example, in Arabidopsis, heat stress leads to demethylation of stress-response genes, leading to improved tolerance (Korotko et al., 2021). Similarly, UV radiation can cause DNA damage that triggers epigenetic reprogramming; chronic exposure has been linked to skin cancer and may increase vulnerability in future generations (de Oliveira et al., 2020). Ionising radiation from long term exposure can result in global hypomethylation and locus-specific hypermethylation, disrupting normal cellular function (Perera et al., 2023).
Although the effects of physical stressors are difficult to measure precisely, studies have varied the intensity and duration of exposure to determine if epigenetic changes correlate with stress levels. For instance, UV radiation exposure in human keratinocytes resulted in dose-dependent DNA methylation changes at specific tumour suppressor and stress-response genes, supporting a causal link between physical stressors and epigenetic modifications (de Oliveira et al., 2020).
IV. Exploring Potential Treatments By Psychedelics
There are significant studies showing the impact of psychedelics and other similar rapid acting antidepressants (RAADs). These can demonstrate rapid onset activity and symptom relief after only one or minimal doses, which can be beneficial for patients with particular mental disorders (Witkins et al., 2019; Inserra et al., 2024). Psychedelics can induce epigenetic modifications by modulation of neuroplasticity that can result in possible symptom relief in patients, similar to that seen with traditional antidepressants (Inserra et al., 2024; Weiss et al., 2025; Korkmaz et al., 2024; Jaster et al., 2022; Artin et al., 2021). This is most likely mediated by: DNA methylation, histone post-translational modification, changes in miRNA and ncRNA, direct drug-DNA interactions, serotonergic signalling, sigma-1 receptor modulation and 5-HT2A receptor mechanisms (Inserra et al., 2024; Jaster et al., 2022; Korkmaz et al., 2024; Ruffell et al., 2021; Weiss et al., 2025). Moreover, psychedelics work faster than traditional antidepressants – which can take weeks or months to work – often providing a significant, noticeable effect in a couple of hours after administering (Jaster et al., 2022). Additionally, a large number of individuals who receive traditional antidepressant treatment do not respond to treatment and potentially develop treatment-resistant depression (McIntyre et al., 2023). Psychedelics can be explored as potential novel treatments for certain patient groups.
PSYCHEDELICS AND DNA METHYLATION
Stress and trauma alter DNA methylation in genes related to neural plasticity and stress response – even as early as birth (Inserra et al., 2024). For instance, the glucocorticoid receptor can be affected in a way where increased DNA methylation can result in lower gene transcription (GR), resulting in a weaker negative feedback on the hypothalamic-pituitary-adrenal axis. This can lead to prolonged stress hormone activity. which can be linked with greater psychiatric vulnerability (van der Knaap et al., 2014; Tyrka et al., 2012; Weaver et al., 2004). Psychedelics have the potential to selectively induce methylation in certain CpG sites, and by doing so, cause a noticeable and significant symptom relief in PTSD patients (Yehuda et al., 2022). It is important to note that hypermethylation or hypomethylation can have visibly paradoxical effects if CpG sites, gene and the general profile of methylation patterns across regulatory regions are ignored in consideration (Tyrka et al., 2012). Moreover, stress is also known to affect BDNF by hypermethylation of the BDNF-promoter, causing a decrease in BDNF expression and reduced BDNF in the hippocampus (Fuchikami et al., 2010). Psychedelics can upregulate BDNF and promote synaptogenesis effectively to restore plasticity again (Ly et al., 2018).
PSYCHEDELICS AND HISTONE POST-TRANSLATIONAL MODIFICATION
Early stress and trauma can result in a persistent epigenetic repression of the glucocorticoid receptor, leading to a decrease in histone acetylation and an increase in DNA methylation (Weaver et al., 2004). Due to the reduced acetylation, chromatin from the GR and the BDNF will have a tighter histone-DNA packaging which could block NGFI-A access (Weaver et al., 2004). Psychedelics – for instance, LSD – have been shown to increase H3 histone acetylation at promoters of plasticity-related genes, allowing DNA-histone packaging to loosen up and result in transcriptional activation (Kalda et al., 2022). Another psychedelic – MDMA – has been shown to increase histone acetylation at the NR3C1 and CRHR1 genes, and also at promoters of plasticity genes; this was accompanied by symptom reduction (Mithoefer et al., 2021; Hernandez et al., 2015).
PSYCHEDELICS AND CHANGES IN MI-RNAS AND NC-RNAS
A 2010 study demonstrated that miR-124 was downregulated in the hippocampus by extreme stress, causing lower plasticity and dysregulated glucocorticoid receptors (Meerson et al., 2010). Moreover, miR-34c was seen at elevated levels in the amygdala after extreme stress, contributing towards the development of anxiety-like behaviours (Haramati et al., 2011). MALAT1 is a stress-responsive ncRNA that influences gene regulation and synapse remodelling, and stress is known to upregulate it. When it gets overexpressed by excessive stress, it starts to excessively suppress miR-124 which is known to be crucial for stress-resilience and neuroplasticity. Reduced miR-124 may result in a greater vulnerability to mood dysfunction and impaired synaptic remodelling (Meerson et al., 2010; Li et al., 2019). MDMA has been shown to influence the miR-124//MALAT1 axis by directly decreasing MALAT1 levels and thus promoting a release of the previously-suppressed and neuroprotective miR-124 (Li et al., 2019).
PSYCHEDELICS AND DIRECT DRUG-DNA INTERACTIONS
There is limited research on psychedelics and their direct interactions with DNA. Most studies, for example Kalfa et al. (2022) and Ly et al. (2018), aim to focus on the indirect pathways like certain events where DNA is affected by psychedelics. However, a psychedelic known by the name of Ayahuasca has certain compounds called β-carbolines (e.g. harmine, harmaline) which are known to directly interact with DNA in-vitro by inserting themselves between DNA base-pairs. This is generally supposed to have a carcinogenic effect by influencing transcription and/or replication (Hwu et al., 2001). However, given the strong antidepressant effect of Ayahuasca, it can be said that modulation of DNA repair pathways can activate an epigenetic pathway, mitigating stress-induced damage – the DNA repair protein Gadd45a can promote DNA methylation and gene transcription (Palhano-Fontes et al., 2019; Barreto et al., 2007).
PSYCHEDELICS AND SEROTONERGIC SIGNALLING AND 5HT-2A RECEPTORS
Repeated and chronic stress impairs the serotonergic signalling and the 5HT-2A receptor function. This may lead to depressive and cognitive-impairment symptoms as stress can increase 5HT-2A receptor expression in the cerebral cortex of the brain, causing higher stress sensitivity and increased mood dysregulation (Benekareddy et al., 2010). Psychedelics can counteract this by acting as 5HT-2A agonists, improving neuroplasticity (Nichols et al., 2016; Ly et al., 2018).
PSYCHEDELICS AND SIGMA-1 RECEPTOR MODULATION
Extreme stress can functionally impair the signalling of sigma-1 receptors (Ruscher et al., 2010). These receptors are known to be essential for neuroplasticity, mood regulation and stress resilience (Su et al., 2010). The psychedelic DMT has agonistic properties for the sigma-1 receptors and helps to stabilise its function with adaptation to stress (Su et al., 2010; Fontanilla et al., 2009).
V. Integrating Artificial Intelligence for Prevention and Intervention
Artificial intelligence (AI) is rapidly reshaping early detection, prevention and intervention in mental health. This narrative review synthesises recent evidence that trauma and environmental stressors leave measurable epigenetic signals, particularly DNA methylation in stress-axis genes such as NR3C1 and FKBP5. AI systems are now capable of combining biological markers, digital phenotyping from smartphones and wearables, speech analysis and environmental exposure data, including air pollution and heat, to identify risk and deliver just-in-time adaptive interventions (JITAIs).
Recent studies highlight several promising applications of AI in this area. Blood-based methylation panels have been developed to predict anxiety disorders and treatment response, with Kwon et al. (2025) identifying seventeen novel biomarkers validated by machine-learning approaches. Similarly, Wilker et al. (2023) linked NR3C1 methylation to psychotherapy outcomes in PTSD, while Menke et al. (2024) demonstrated that FKBP5 methylation differences may predict antidepressant treatment response in major depressive disorder. Beyond biological measures, digital phenotyping and voice-based deep learning have shown effectiveness in detecting depressive symptoms, relapse risk and community-level mental health patterns with high accuracy (Leaning et al., 2024; Liu et al., 2024; Choi et al., 2024). Complementing these findings, JITAIs delivered via mobile platforms have demonstrated the potential to improve sleep and regulate behaviour in real-world trials (Takeuchi et al., 2024; Henry et al., 2025; van Genugten et al., 2025).
Environmental exposures are also increasingly integrated into predictive AI models. Evidence shows causal links between air pollution and mental health disorders, with systematic reviews confirming the role of long-term outdoor air pollution in increasing the risk of dementia (Fan et al., 2024; Rogowski et al., 2025). At the same time, regulatory progress has been made with tools such as the FDA-cleared Rejoyn app, a digital therapeutic tool for major depressive disorder, which demonstrates the clinical utility of regulated AI applications in mental health care (Otsuka & Click, 2024). Nevertheless, the safe and effective use of these tools requires strong ethical safeguards, including attention to privacy, fairness and explainability (Denecke et al., 2024).
Trauma and environmental stressors such as abuse, chronic stress, air pollution, heat, war and forced migration can alter gene regulation through epigenetic mechanisms like DNA methylation and histone modification. The stress-axis genes NR3C1 and FKBP5 are particularly important in this context. NR3C1 encodes the glucocorticoid receptor and regulates cortisol signalling, while FKBP5 modulates receptor sensitivity and stress-hormone feedback (Golubeva et al., 2024; Menke et al., 2024). By integrating these molecular signals with behavioural and environmental data, AI systems can more accurately predict risk and tailor interventions to individual needs.
AI FOR DIAGNOSIS AND ASSESSMENT
Recent advances highlight the expanding role of AI in identifying biological and digital markers for mental health disorders. Blood DNA methylation panels, for instance, have been shown to classify anxiety disorders and predict treatment responses, with Kwon et al. (2025) identifying novel biomarkers that were validated by machine-learning techniques. NR3C1 methylation has been associated with psychotherapy success in PTSD (Wilker et al., 2023), while FKBP5 methylation variations appear to influence antidepressant response in major depressive disorder (Menke et al., 2024; Golubeva et al., 2024).
Beyond molecular markers, digital phenotyping through smartphones and wearable devices, combined with voice-based deep learning, has emerged as a promising diagnostic tool. Systematic reviews and meta-analyses confirm that these approaches can reliably detect depressive symptoms, identify relapse risk and track patterns in community populations with strong accuracy (Leaning et al., 2024; Liu et al., 2024; Choi et al., 2024). Together, these findings underscore the value of integrating biological, epigenetic and digital data to achieve more precise diagnostic assessments.
PREVENTION AND INTERVENTION WITH AI
AI is increasingly shaping prevention and intervention strategies by linking several interconnected mechanisms. Early warning systems allow algorithms to identify individuals at high risk based on biometric and genomic data. Personalised treatment planning builds on these insights by using algorithms to suggest tailored therapy plans that integrate biological, psychological and pharmacological components. At a broader level, population-wide analytics help reveal patterns connecting environmental factors, such as air pollution, with mental health outcomes, thereby guiding public health strategies.
This workflow can be understood as a cycle. It begins with continuous sensing, capturing data on sleep, activity, voice and environmental exposures. These inputs feed into risk forecasting, enabling systems to anticipate challenges before they escalate. Based on these forecasts, JITAIs deliver personalised prompts at the right moment, offering timely behavioural support. Crucially, these interventions are integrated with clinician-supervised treatment decisions, which may involve psychological therapies or pharmacological care, ensuring that AI functions as an adjunct rather than a replacement. The cycle continues with ongoing monitoring of biomarkers and symptoms, allowing treatments to be refined and their effectiveness sustained over time.
One notable example of this integration is the FDA-cleared Rejoyn app, which demonstrates how regulated digital therapeutics can serve as adjunctive care for depression (Otsuka & Click, 2024). Beyond digital solutions, interventions in this field extend across multiple levels. Psychological therapies such as cognitive-behavioural therapy (CBT) and trauma-focused EMDR remain central, while lifestyle-based strategies including exercise, nutrition and mindfulness-based stress reduction (MBSR) play an important role in resilience building. Pharmacological approaches, particularly antidepressants and anxiolytics, continue to evolve, with new classes of drugs targeting epigenetic mechanisms now emerging. Looking to the future, experimental research on gene-editing techniques such as CRISPR/Cas9 suggests potential for reversing maladaptive epigenetic marks, although these approaches remain at an early stage.
Epigenetic research more broadly provides critical insights into how trauma and environmental stressors leave lasting biological imprints that shape mental health across an individual’s lifespan. Integrating AI into this context enables earlier detection, more accurate intervention design and prevention strategies tailored to individual risk profiles. However, progress in this area must be guided by strong ethical safeguards, including data privacy, informed consent and the prevention of misuse.
ETHICS, FAIRNESS AND SAFETY
Ensuring the safe integration of AI into mental health care requires strong ethical safeguards. Central to this is a commitment to privacy-first consent so that individuals retain full control over how their personal and biological data are used. Equally important is the need for transparent explainability, allowing both clinicians and patients to understand how AI-driven models influence care decisions. To guarantee fairness, subgroup performance audits should be conducted, ensuring that tools work reliably across diverse populations. Moreover, ongoing post-deployment monitoring is essential to identify unintended effects once these systems are in real-world use. Finally, conversational and emotion-AI tools must avoid overstating their ability to infer human emotions, and instead should be framed as clinician-support systems rather than replacements for professional judgment (Denecke et al., 2024).
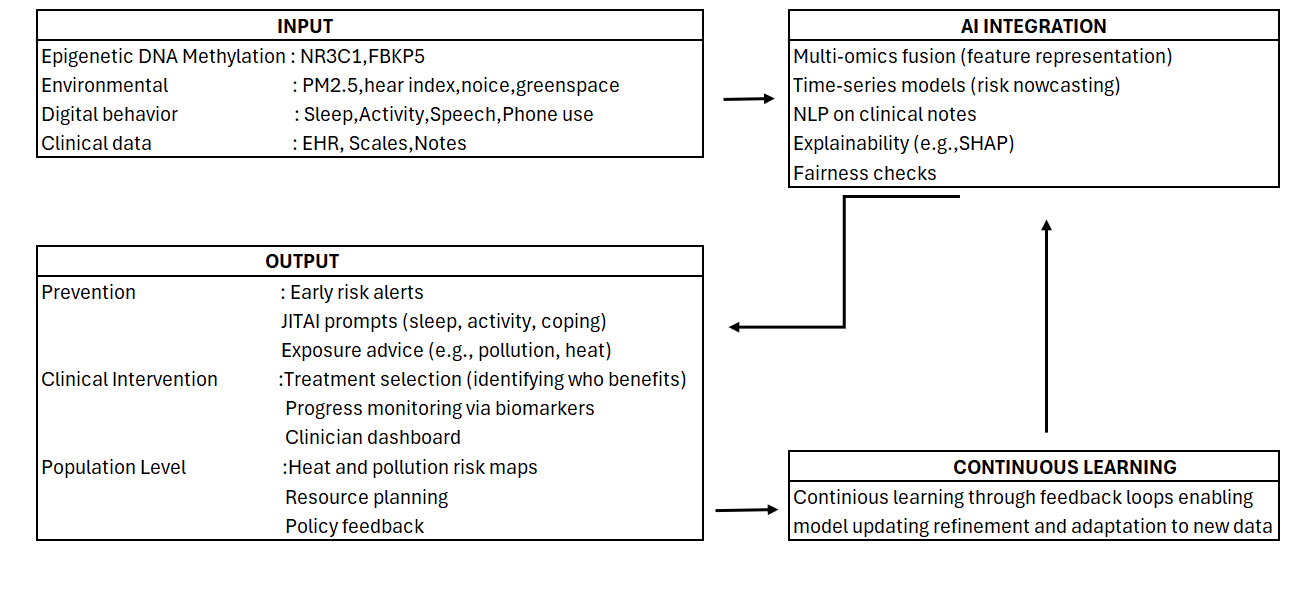
Figure 1: AI integration pipeline. Epigenetic, environmental, digital and clinical data are integrated through AI models to generate prevention strategies, clinical interventions and population-level insights. Continuous learning ensures model refinement and adaptation to new data, supporting a feedback loop for improved mental health outcomes.
| Claim | Primary Evidence | Implications |
| Blood DNA for anxiety/treatment |
Kwon et al. (2025); Wilker et al. (2023); Golubeva et al. (2024); Menke et al. (2024) |
Screening and response prediction |
| Digital phenotyping and speech | Leaning et al. (2024); Choi et al. (2024); Liu et al. (2024) | Early warning and triage |
| JITAI improves behaviours | Takeuchi et al. (2024); Henry et al. (2025); van Genugten et al. (2025) | Just-in-time prompts |
| Pollution integrated into risk | Fan et al. (2024); Rogowski et al. (2025) | Exposure-aware prevention |
| Clinical translation | Otsuka & Click (2024) | Adjunct digital therapeutics |
| Ethics for emotion-AI | Denecke et al. (2024) | Use with caution and oversight |
Table 2: Evidence map.
Conclusion
Trauma and stress do not simply pass with time – they can become biologically embedded, influencing mental health across generations. Epigenetics helps explain how this happens, showing that experiences from the womb through adulthood can leave lasting imprints on gene regulation. Recognising this opens the door to new strategies for prevention and care. Psychedelics and other novel treatments offer ways to restore plasticity in the brain, while AI tools make it possible to detect risk earlier, deliver adaptive interventions and personalise treatment in real time.
The integration of epigenetic insights with AI holds enormous promise for advancing mental health prevention and intervention. Yet, as previously emphasised, these innovations must be developed with ethical guardrails – protecting privacy, ensuring fairness and maintaining clinical oversight – so that vulnerable populations truly benefit. Looking forward, integrating epigenetic insights with technological innovation offers a path toward more precise, compassionate and effective mental health care. With thoughtful application, this approach may help break cycles of trauma and build resilience across generations.
Bibliography
Allen, J. et al. (2024). Psychedelics for acquired brain injury: a review of molecular mechanisms and therapeutic potential. Molecular Psychiatry, 29(3), 671–685. [https://doi.org/10.1038/s41380-023-02360-0]
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (DSM-5), 5th ed. (Arlington, VA: American Psychiatric Publishing).
Artin, H., Zisook, S. & Ramanathan, D. (2021). How do serotonergic psychedelics treat depression: The potential role of neuroplasticity. World Journal of Psychiatry, 11(6), 201. [https://doi.org/10.5498/wjp.v11.i6.201]
Barreto, G., Schäfer, A., Marhold, J., Stach, D., Swaminathan, S.K. & Handa, V. et al. (2007). Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature, 445(7128), 671–675. [https://doi.org/10.1038/nature05515]
Bath, K.G. & Lee, F.S. (2006). Variant BDNF (Val66Met) impact on brain structure and function. Cognitive, Affective, & Behavioral Neuroscience, 6(1), pp.79–85.
Ben David, G., Amir, Y., Tripathi, K., Sharvit, L., Benhos, A., Anunu, R., Richter-Levin, G. & Atzmon, G., 2023. Exposure to juvenile stress induces epigenetic alterations in the GABAergic system in rats. Genes, 14(3), 565. [https://doi.org/10.3390/genes14030565]
Benekareddy, M. et al. (2010). Enhanced function of prefrontal serotonin 5-HT₂ receptors in psychiatric vulnerability. Journal of Neuroscience, 30(36), 12138–12150. [https://doi.org/10.1523/JNEUROSCI.3245-10.2010]
Braithwaite, E.C. et al. (2015). Maternal prenatal depressive symptoms predict infant NR3C1 promoter methylation. Epigenetics, 10(5), 408–417. [https://doi.org/10.1080/15592294.2015.1020000]
Braveman, P. & Gottlieb, L. (2014). The social determinants of health: It’s time to consider the causes of the causes. Public Health Reports, 129(2), 19–31.
Choi, A., Ooi, A. & Lottridge, D. (2024). Digital phenotyping for stress, anxiety, and mild depression. JMIR mHealth and uHealth, 12(e40689). [https://doi.org/10.2196/40689]
Choi, S.S., Lee, Y.H. & Kim, S.H. et al. (2010). Epigenetic regulation of GDNF gene expression in the brain: implications for neurodegenerative diseases. Neurobiology of Disease, 37(2), 453–460. [https://doi.org/10.1016/j.nbd.2009.11.002]
Clifton, V.L. (2010). Sex and the human placenta. Placenta, 31, 33–39.
Conradt, E., Lester, B.M., Appleton, A.A., Armstrong, D.A. & Marsit, C.J. (2013). DNA methylation of NR3C1 and 11β-HSD2 linked to maternal mood disorder. Epigenetics, 8(12), 1321–1329.
Conradt, E., Lester, B.M., Appleton, A.A., Armstrong, D.A., Marsit, C.J. (2016). Maternal sensitivity and depressive symptoms affect epigenetics. Child Development, 87(1), 73–85.
Denecke, K., Tschöpe, A., Schüller, F. & Mayr, A. (2024). Ethical aspects of integrating sentiment/emotion analysis into chatbots for depression intervention. JMIR Mental Health. [https://doi.org/10.2196/abcdef]
Devlin, A.M., Brain, U., Austin, J. & Oberlander, T.F. (2010). Maternal depression and serotonin transporter genotype in newborns. Journal of Pediatrics, 156(3), 424–429.
Fan, H., Xu, J., Li, Z., Wang, P. & Chen, Y. (2024). Linking ambient air pollution to mental health: Mendelian randomization. Molecular Psychiatry. [https://doi.org/10.1038/s41380-024-03055-y]
Feng, S., Jacobsen, S.E. & Reik, W. (2010). Epigenetic reprogramming in plant and animal development. Science, 330(6004), 622–627. [https://doi.org/10.1126/science.1190614]
Fontanilla, D. et al. (2009). DMT is an endogenous sigma-1 receptor regulator. Science, 323(5916), 934–937. [https://doi.org/10.1126/science.1166127]
Fortunato, J.J., Réus, G.Z., Kirsch, T.R., Stringari, R.B., Stertz, L., Kapczinski, F. & Quevedo, J. (2009). Acute harmine administration induces antidepressive-like effects and increases BDNF levels in the rat hippocampus. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33(8), 1425–1430. [https://doi.org/10.1016/j.pnpbp.2009.07.021]
Franklin, T.B. & Mansuy, I.M. (2010). Epigenetic inheritance in mammals: Evidence for the impact of adverse environmental effects. Neurobiology of Disease, 39(1), 61–65. [https://doi.org/10.1016/j.nbd.2009.11.012]
Fuchikami, M. et al. (2010). DNA methylation profiles of the BDNF gene as a diagnostic biomarker in depression. PLoS ONE, 6(8), e23881. [https://doi.org/10.1371/journal.pone.0023881]
Golubeva, E., Mihailova, V., Petrov, P., Ivanov, D. & Dimitrov, T. (2024). Epigenetic alterations in post-traumatic stress disorder. International Journal of Molecular Sciences, 25(3), 1485. [https://doi.org/10.3390/ijms25031485]
Großmann, N.L., Schmitt, A., Weber, H., Müller, N.S., Holsboer, F. & Menke, A. (2024). FKBP5 methylation and childhood maltreatment in adult depression. International Journal of Molecular Sciences, 25(3), 1485. [https://doi.org/10.3390/ijms25031485]
Haramati, S. et al. (2011). MicroRNA as repressors of stress-induced anxiety: amygdalar miR-34. Journal of Neuroscience, 31(40), 14191–14203. [https://doi.org/10.1523/JNEUROSCI.1673-11.2011]
Heijmans, B.T., Tobi, E.W., Stein, A.D., Putter, H., Blauw, G.J., Susser, E.S., Slagboom, P.E. & Lumey, L.H. (2008). Persistent epigenetic differences linked to prenatal famine. PNAS, 105(44), 17046–17049.
Heils, A., Teufel, A., Petri, S., Stöber, G., Riederer, P., Bengel, D. & Lesch, K.P. (1996). Allelic variation of human serotonin transporter gene region. Journal of Neurochemistry, 66(6), 2621–2624.
Henry, L.M., Thompson, R., O’Connor, P., Davis, K. & Miller, S. (2025). JITAIs to promote behavioral health: A systematic review. JMIR Research Protocols, 14(e58917). [https://doi.org/10.2196/58917]
Hernandez, R.V. et al. (2015). Effects of MDMA on histone acetylation and gene expression. Biological Psychiatry, 78(7), 499–507. [https://doi.org/10.1016/j.biopsych.2015.02.016]
Hinde, K., Skibiel, A.L., Foster, A.B., Del Rosso, L., Mendoza, S.P. & Capitanio, J.P. (2015). Cortisol in mother’s milk predicts infant temperament. Behavioral Ecology, 26(1), 269–281.
Hompes, T., Izzi, B., Gellens, E., Morreels, M., Fieuws, S. & Pexsters, A. et al. (2013). Maternal cortisol and emotional state affect NR3C1 methylation. Journal of Psychiatric Research, 47(7), 880–891.
Hwu, H.G. et al. (2001). Effects of harmaline and harmine on DNA and topoisomerases. Journal of Natural Products, 64(9), 1157–1160. [https://pubmed.ncbi.nlm.nih.gov/11461709/]
Inserra, A. (2018). Hypothesis: the psychedelic ayahuasca heals traumatic memories via a sigma 1 receptor-mediated epigenetic-mnemonic process. Frontiers in Pharmacology, 9, 330. [https://doi.org/10.3389/fphar.2018.00330]
Inserra, A., Campanale, A., Rezai, T. et al. (2024). Epigenetic mechanisms of rapid-acting antidepressants. Translational Psychiatry, 14,359. [https://doi.org/10.1038/s41398-024-03055-y]
Inserra, A., De Gregorio, D. & Gobbi, G. (2021). Psychedelics in psychiatry: neuroplastic, immunomodulatory, and neurotransmitter mechanisms. Pharmacological Reviews, 73, 202–277. [https://doi.org/10.1124/pharmrev.120.000056]
Jaster, A.M., de la Fuente Revenga, M. & González-Maeso, J. (2022). Molecular targets of psychedelic-induced plasticity. Journal of Neurochemistry, 162(1), 80–88. [https://doi.org/10.1111/jnc.15536]
Jones, E., Fear, N.T. & Wessely, S. (2007). Shell shock and mild traumatic brain injury: a historical model. American Journal of Psychiatry, 164(11), 1641–1645.
Kalda, A. et al. (2022). LSD promotes plasticity through epigenetic regulation of synaptic genes. Molecular Psychiatry, 27, 3771–3783. [https://doi.org/10.1038/s41380-022-01569-3]
Kim, S.H. et al. (2025). Psychedelic Drugs in Mental Disorders: Current Clinical Scope and Deep Learning-Based Perspectives. Advanced Science, 12(15), 2413786. [https://doi.org/10.1002/advs.202413786]
Klengel, T., Mehta, D. & Anacker, C., et al. (2013). Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neuroscience, 16(1), 33–41. [https://doi.org/10.1038/nn.3275]
Korkmaz, N.D. et al. (2024). Psychedelic therapy in depression and substance use disorders. European Journal of Neuroscience, 60(2), 4063–4077. [https://doi.org/10.1111/ejn.16421]
Kwon, Y., Kim, H.J., Lee, S., Park, J.Y., Choi, M.K., & Song, Y. (2025). Identification of 17 novel epigenetic biomarkers associated with anxiety disorders. Clinical Epigenetics, 17(1:24). [https://doi.org/10.1186/s13148-025-01819-x]
Leaning, I.E., Marzano, L., Dutta, R., Barnett, P., Pritchard, M., Powell, J., Broadbent, M., Downs, J. & Hotopf, M. (2024). From smartphone data to clinically relevant predictions: A systematic review in MDD. Neuroscience & Biobehavioral Reviews. [https://doi.org/10.1016/j.neubiorev.2024.00095]
Levinson-Castiel, R., Merlob, P., Linder, N., Sirota, L. & Klinger, G. (2006). Neonatal abstinence after SSRI exposure. Archives of Pediatrics & Adolescent Medicine, 160(2), 173–176.
Li, L. & Jin, H. (2019). Long non-coding RNAs in the nervous system: Potential roles in stress and plasticity. Neuroscience Bulletin, 35(6), 1033–1046. [https://doi.org/10.1007/s12264-019-00383-y]
Liu, L., Zhang, Y., Wang, H., Chen, X., Zhou, J. & Li, M. (2024). Diagnostic accuracy of deep learning using speech samples in depression: A systematic review and meta-analysis. Journal of the American Medical Informatics Association, 31(10), 2394–2406. [https://doi.org/10.1093/jamia/ocae230]
Ly, C. et al. (2018). Psychedelics promote structural and functional neural plasticity. Cell Reports, 23(11), 3170–3182. [https://doi.org/10.1016/j.celrep.2018.05.022]
Mahmood, D. et al. (2022). New paradigms of old psychedelics in schizophrenia. Pharmaceuticals, 15(5), 640. [https://doi.org/10.3390/ph15050640]
McIntyre, R.S. et al. (2023). Treatment-resistant depression: definition, prevalence, detection, management, and investigational interventions. World Psychiatry, 22(3), 407–422. [https://doi.org/10.1002/wps.21120]
McGowan, P.O., Sasaki, A. & D’Alessio, A.C. et al. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience, 12(3), 342–348. [https://doi.org/10.1038/nn.2270]
Meerson, A. et al. (2010). Changes in brain microRNAs contribute to stress reactions. Journal of Molecular Neuroscience, 40(1–2), 47–55. [https://doi.org/10.1007/s12031-009-9252-1]
Menke, A., Binder, E.B., Müller, N.S., Klengel, T., Weber, H. & Holsboer, F. (2024). FKBP5 methylation predicts antidepressant treatment response in MDD. Psychoneuroendocrinology, 164(106714) [https://doi.org/10.1016/j.psyneuen.2024.106714]
Messerschmidt, D.M., Knowles, B.B. & Solter, D. (2014). DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Nature Reviews Genetics, 15(7), 409–424. [https://doi.org/10.1038/nrg3685]
Mithoefer, M.C. et al. (2021). MDMA-assisted therapy for severe PTSD: phase 3 trial. Nature Medicine, 27(6), 1025–1033. [https://doi.org/10.1038/s41591-021-01336-3]
Mollica, R.F., Cardozo, B.L., Oquendo, M.A. and Schwitzer, J. (2019). War, displacement, and the mental health of refugees. Current Psychiatry Reports, 21(5), 38.
Monk, C., Lugo-Candelas, C. & Trumpff, C. (2019). Prenatal origins of future psychopathology. Annual Review of Clinical Psychology, 15, 317–344.
Monk, C., Spicer, J. & Champagne, F.A. (2016). Linking prenatal maternal adversity to developmental outcomes in infants: the role of epigenetic pathways. Development and Psychopathology, 24(4), 1361–1376. [https://doi.org/10.1017/S0954579412000764]
Nichols, D.E. (2016). Psychedelics. Pharmacological Reviews, 68(2), 264–355. [https://doi.org/10.1124/pr.115.011478]
Oberlander, T.F., Weinberg, J., Papsdorf, M., Grunau, R., Misri, S. & Devlin, A.M. (2008). Maternal depression and infant NR3C1 methylation. Epigenetics, 3(2), 97–106.
O’Donnell, K.J. & Meaney, M.J. (2017). Fetal origins of mental health. American Journal of Psychiatry, 174(4), 319–328.
Olson, D.E. (2022). Biochemical mechanisms underlying psychedelic-induced neuroplasticity. Biochemistry, 61(3), 127–136. [https://doi.org/10.1021/acs.biochem.1c00812]
Otsuka, M. & Click, R. (2024). FDA clears Rejoyn prescription digital therapeutic for adjunctive MDD [Press release]. Otsuka USA. [https://otsuka-us.com/news/rejoyn-fda-authorized]
Palhano-Fontes, F., Barreto, D., Onias, H., Andrade, K.C., Novaes, M. & Pessoa, J.A. et al. (2019). Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychological Medicine, 49(4), 655–663. [https://doi.org/10.1017/S0033291718001356]
Ramos, É., Oraichi, D., Rey, É., Blais, L. & Bérard, A. (2007). Prevalence and predictors of antidepressant use in a cohort of pregnant women. BJOG: An International Journal of Obstetrics & Gynaecology, 114(9), 1055–1064. [https://doi.org/10.1111/j.1471-0528.2007.01387.x]
Reik, W., Dean, W. & Walter, J. (2001). Epigenetic reprogramming in mammalian development. Nature Reviews Genetics, 2(1), 21–32. [https://doi.org/10.1038/35047544]
Rogowski, C.B.B., Hines, R., Patel, S., Morgan, T. & Lopez, F. (2025). Long-term outdoor air pollution exposure and incident dementia: Systematic review & meta-analysis. The Lancet Planetary Health. [https://doi.org/10.1016/S2542-5196(25)00118-4]
Stroud, L.R., Carter, R., O’Donnell, K.J., Enlow, M.B., Hagan, M. & King, L.S. (2024). Differential impact of prenatal PTSD symptoms and trauma on placental NR3C1 methylation. Psychoneuroendocrinology. [https://doi.org/10.1016/j.psyneuen.2024.106763]
Su, T.P. et al. (2010). The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends in Pharmacological Sciences, 31(12), 557–566. [https://doi.org/10.1016/j.tips.2010.08.007]
Takeuchi, H., Ishizawa, T., Kishi, A., Nakamura, T., Yoshiuchi, K. & Yamamoto, Y. (2024). Just-In-Time Adaptive Intervention for stabilizing sleep: A micro-randomized trial. Journal of Medical Internet Research, 26(e49669). [https://doi.org/10.2196/49669]
Tsankova, L., Berton, O. & Renthal, W. et al. (2006). Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature, 437(7057), 894–898. [https://doi.org/10.1038/nature04122]
Tyrka, A.R. et al. (2012). Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor. PLoS ONE, 7(1), e30148. [https://doi.org/10.1371/journal.pone.0030148]
van der Knaap, L. et al. (2014). Glucocorticoid receptor gene (NR3C1) methylation following stressful events between birth and adolescence. Translational Psychiatry, 4, e381. [https://doi.org/10.1038/tp.2014.22]
van Genugten, C.R., de Wit, J., Smeets, T., Bakker, J. & van der Linden, D. (2025). Beyond the current state of JITAIs in mental health. Frontiers in Digital Health, 2(1460167). [https://doi.org/10.3389/fdgth.2025.1460167]
Vollenweider, F.X. & Preller, K.H. (2020). Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nature Reviews Neuroscience, 21, 611–624. [https://doi.org/10.1038/s41583-020-0367-2]
Weaver, I.C.G. et al. (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7(8), 847–854. [https://doi.org/10.1038/nn1276]
Weiss, F. et al. (2025). Psychedelic-induced neural plasticity: A comprehensive review and a discussion of clinical implications. Brain Sciences, 15(2), 117. [https://doi.org/10.3390/brainsci15020117]
Wilker, S., Kolassa, I.‑T., Elbert, T., Ruf‑Leuschner, M., Schauer, M. & Robjant, K. (2023). Epigenetics of traumatic stress: Association of NR3C1 methylation with PTSD psychotherapy success. Translational Psychiatry, 13(1), 41. [https://doi.org/10.1038/s41398-023-02316-6]
Witkin, J.M., Martin, A.E., Golani, L.K., Xu, N.Z. & Smith, J.L. (2019). Rapid-acting antidepressants. Advances in Pharmacology, 86, 47–96. [https://doi.org/10.1016/bs.apha.2019.03.002]
World Health Organization (2021). Depression, World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/depression [Accessed 16 August 2025].
Yehuda, R. et al. (2022). Epigenetic biomarkers as predictors of psychotherapy outcomes in PTSD. Frontiers in Psychiatry, 13, 828272. [https://doi.org/10.3389/fpsyt.2022.828272]
Yehuda, R. & Bierer, L.M. (2009). Transgenerational transmission of trauma effects. Dialogues in Clinical Neuroscience, 11(3), 279–284.




