Abstract
Nutrition across the human lifespan has significant effects on health, not only through direct physiological pathways but also through epigenetic mechanisms that regulate gene expression without directly altering DNA sequences. This paper reviews how variations in macro- and micronutrient intake during critical developmental windows (pregnancy, early childhood and puberty), shape long-term health outcomes and disease risk in adulthood. We discuss how nutrient deficiencies and excesses influence foetal growth, cognitive and physical development, as well as the age of puberty onset due to the consequences of DNA methylation, histone modification and non-coding RNAs. Case studies, such as the Dutch Hunger Winter, and experimental models, such as the Agouti mouse, illustrate how early nutritional exposures leave persistent epigenetic marks that predispose individuals to conditions such as cardiovascular disease, type 2 diabetes and obesity later in life. We also examine how adult dietary choices can reinforce or mitigate these accumulated epigenetic modifications and how this can potentially have intergenerational effects. Our findings highlight that both undernutrition and overnutrition remain global challenges, affecting populations across socioeconomic contexts. Ultimately, understanding the intersection of nutrition and epigenetics emphasises the importance of balanced diets during all stages of life and underscores the role of public health measures in reducing the long-term and transgenerational consequences of poor nutrition.
Introduction
The rising global issue of metabolic syndrome, obesity and type 2 diabetes all present a growing health crisis (Ahmed & Mohammed, 2025). These issues are all rooted in modern lifestyles and caloric excess. However, it can be suggested that these problems we face are seeded much earlier in life, typically in the most vital periods in development. According to the Developmental Origins of Health and Disease hypothesis, the nutritional intake in utero, infancy and adolescence is the deciding factor in an individual’s long-term physiological trajectory (Gluckman, Hanson & Buklijas, 2010). This occurs through epigenetics, the regulator of gene expression in response to environmental cues. Therefore, nutrients act more like fuel as they are potent epigenetic modifiers that can silence or activate genes with lifelong consequences (Feil & Fraga, 2012).
A woman during pregnancy requires an optimum amount of micronutrients and macronutrients to support the growth and development of the foetus. Yet, dietary intake in pregnant women across all socioeconomic scales falls dangerously short of requirements (Khammarnia et al., 2024). This creates a double burden of malnutrition, which is when undernutrition and macronutrient deficiencies occur with obesity and overnutrition, both are said to be capable of heavily disrupting early developmental pathways (Stephenson et al., 2024). The results extend far beyond pregnancy complications, such as low birth weight and preeclampsia, and embed a higher risk for the offspring to develop hypertension, cardiovascular disease and metabolic dysfunction that may persist well into adulthood (Agustina et al., 2023).
This paper will argue that the balanced intake of micro- and macronutrients in the diet during vital developmental stages plays a determining factor in adult health through the mechanism of epigenetic modification. With sufficient evidence, we will demonstrate how nutrient availability – from deficiency to excess – during pregnancy, early childhood and puberty decides the long-term changes in DNA methylation, non-coding RNA expression and histone acetylation. Through profound examples such as the Agouti mouse model and the Dutch Hunger Winter, we will describe how these nutritional marks alter the expression of genes that control immunity, metabolism and neurodevelopment, thereby predisposing individuals to chronic diseases. Finally, we will explore the dietary interventions for adults to modulate these early life marks while simultaneously considering the disquieting evidence for the transgenerational inheritance of nutritional epigenetic effects. Doing so, this review will highlight that the optimal intake of nutrition from the earliest stages of life is not just for the support of growth, but for other beneficial factors that act as strategies for breaking cycles of chronic diseases and preventive medicine.
Difference in average macro- and micronutrient consumption during pregnancy in different countries and the effects on foetal development
Pregnancy requires increased nutrient intake from the mother to ensure optimal foetal growth. This increase must be in both the intake of macronutrients, such as fats, carbohydrates and proteins, and in micronutrients, such as vitamins and folate. Macronutrients are necessary to provide energy and to support the growth of tissues. However, for many mothers and women of reproductive age, nutrient intake is far below recommendations, which puts both the mother and foetus at risk. Average total energy is below the requirements for pregnancy (Khammarnia et al., 2024), and poor nutrition and obesity are prevalent in women of both high-income and low-income countries alike (Stephenson et al., 2018). Inadequate nutrient intake can cause preeclampsia, low birth weight and increased risks of maternal and infant mortality (Agustina et al., 2023). It can also cause cardiovascular disease, type 2 diabetes and hypertension (Khammarnia et al., 2024). Chronic energy deficiency is also common among pregnant women and women of reproductive age, due to factors such as family size, living in rural areas, insufficient meal frequency, poor diet quality and low socioeconomic status (Khammarnia et al., 2024). This section will discuss the comparisons in macronutrient and micronutrient consumption among different countries and their effects on foetal development.
Protein has both structural and functional roles biologically (Mousa, Naqash & Lim, 2019). The amino acids found in proteins are essential for protein synthesis and the creation of other nitrogenous substances (Herring et al., 2018). Protein consumption during pregnancy has been linked to a lowered risk of low birth weight, small for gestational age (SGA) and intrauterine growth restriction (IUGR) (Yang et al., 2022). IUGR increases the risk of neonatal mortality, with surviving infants experiencing increased risk of developing hormonal imbalances, abnormal development, metabolic disorders and cardiovascular disorders in adulthood (Herring et al., 2018). Analysis has shown that the average total protein intake among pregnant women in different countries was 78.21 g/day. It also indicated a significant difference between the amount of protein consumed in different studies (Khammarnia et al., 2024).
Carbohydrates should account for half of the energy intake of pregnant women (Xue et al., 2024), providing glucose for both foetal brain development and the placenta (Hernandez & Rozance, 2023). Restricted consumption of carbohydrates has been linked to increased risk of T2DM, while increased consumption has been shown to decrease risk for impaired glucose tolerance and GDM (Xue et al., 2024). However, the quality of carbohydrates is more notable in the effect on pregnant women and children. Several studies suggest that increasing the consumption of whole grains, vegetables, legumes and lean meats, while reducing the consumption of highly-processed foods, may be beneficial (Xue et al., 2024). Higher maternal carbohydrate intake was inversely associated with the fat index of both the mother and the newborn (Kizirian et al., 2016).
“Folates” is a general term including folic acid and its derivative compounds, the former of which is a well-known epigenetic modifier (Socha, Flis & Wartega, 2024). It is a water-soluble B vitamin present in leafy greens, yeast extract and citrus fruit (Mousa, Naqash & Lim, 2019). Numerous studies indicate a relationship between folic acid deficiency and the occurrence of neural tube defects (Socha, Flis & Wartega, 2024), though no effects have been found on miscarriage or other birth defects (Mousa, Naqash & Lim, 2019). Folic acid deficiency may also be associated with placental abruption and preeclampsia. In contrast, folic acid supplementation is associated with a reduction in the occurrence of IUGR (Socha, Flis & Wartega, 2024). There is also an increased risk of autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) in offspring. This is possibly because folic acid deficiency may translate into insufficient production of methyl donors, which leads to alterations in DNA methylation patterns, silencing certain genes and ultimately leading to neurodevelopmental disorders (Socha, Flis & Wartega, 2024).
Calcium is necessary for human bone maintenance and development, and it cannot be manufactured by the body. It is especially important during pregnancy; if adequate bone is not built before pregnancy and adequate calcium is not part of the mother’s diet, bone can be degraded as calcium is taken from the mother’s skeleton (Thomas & Weisman, 2006). At the same time, only 6% of pregnant women consume the recommended daily amount of calcium, with actual daily intakes closer to 50-70% of what is recommended (Thomas & Weisman, 2006).
The effects of macro- and micronutrient consumption on early childhood development
There are four main stages of early childhood development: babies, toddlers, pre-school and grade school, all of which are separated by their own key characteristics and abilities in the areas of cognitive, motor, language, communication, social and emotional development (Cleveland Clinic Child Development, 2022). The first years of life, typically until the age of 8, represent the most important window for cognitive development and human growth (Cleveland Clinic Child Development, 2022). This is significant as during this period, physical maturation is directly influenced by nutritional intake. Other factors such as immunity, neurological function and metabolic rates continue to persist throughout the child’s lifetime and sometimes continue into future generations through epigenetic mechanisms (Gluckman, Hanson & Buklijas, 2010). Epigenetics is the study of gene changes that are heritable and that do not require alterations to underlying DNA sequences. This includes processes such as DNA methylation and histone modification, which are directly influenced by various environmental factors such as stress, diet and toxins. The field of epigenetics provides a framework for the understanding of how early nutritional exposure can build prominent long-term outcomes (Fiel & Fraga, 2012). In this section, we discuss the roles of micronutrients (vitamins and minerals) and macronutrients (protein, fats and carbohydrates) in early development, the deficiency disorders that are associated with them and the epigenetic modifications they induce, which ultimately may collectively shape the child’s susceptibility to chronic diseases in adulthood.
Proteins – a well-known foundation to enzymatic reactions and cellular structure, and responsible for signalling hormones – are nearly indispensable for immune function and early development (WHO, 2020). If the optimum threshold of 9.1g-11g for sufficient protein intake is not reached during infancy, it can lead to protein-energy malnutrition (PEM), which can be seen through wasting (low weight for age), stunting (low height for age) and kwashiorkor (characterised by oedematous malnutrition) (Black et al., 2013). Furthermore, a deficiency of protein may impair neurotransmitter synthesis, specifically in dopamine and serotonin, which are both critical to cognitive and behavioural development in a child (Georgieff, 2007). In addition, protein malnutrition affects the methylation patterns in the DNA of metabolic genes that influence insulin-like growth factor 1 (IGF1), responsible for regulating differentiation and cellular growth (Waterland & Jirtle, 2003). Animal studies by Lillycrop et al. restricted protein from the diets of pregnant rats and demonstrated hypomethylation due to prenatal protein restriction of IGF1, leading to increased risk of metabolic syndrome in adulthood and dysregulated growth patterns (Lillycrop et al., 2005). Additionally, the amino acid methionine serves as a methyl donor for histone methylation and DNA, indicating that protein deficiency can disrupt global epigenetic regulation (Niculescu & Zeisel, 2002).
Dietary fats, specifically the long-chain polyunsaturated fatty acids such as docosahexaenoic acid (DHA) and arachidonic acid (AA), are also significant for the development of the neural region. As a result, they constitute over 60% of the brain’s weight dry (Innis, 2008). AA serves as a precursor for inflammatory mediators, while DHAs are incoporated into neuronal membranes, enhancing signal transduction and synaptic plasticity (Dyall, 2015). An example of a polyunsaturated fat is omega-3, found in foods such as fish and flaxseed. Insufficient intake of omega-3 during infancy and early childhood can correlate with traits such as reduced IQ, visual acuity and a higher risk of ADHD (McCannes & Ames, 2005). Furthermore, it was also found in the ALSPAC Cohort Study that children with lower DHA levels also showed persistent behavioural disorders and delayed language acquisition (Hibblen et al., 2007). DHA also acts as a modulator for histone acetylation at the brain-derived neurotrophic factor (BDNF) promoter, where cognitive function and neural survival are dramatically enhanced (Crupi et al., 2013). In addition, maternal deficiency in omega-3 levels leads to hypomethylation of glucocorticoid receptors, which can increase the risk of the offspring developing stress-related psychiatric disorders (Vucatic et al., 2010).
Dietary carbohydrates are typically the main source of energy for the body, roughly accounting for over half of the body’s glucose in early childhood (Prado & Dewey, 2014). However, the overeating of refined sugar is linked to obesity, insulin resistance and non-alcoholic fatty liver disease (Ludwig & Ebbeling, 2018). Moreover, DNA methylation changes in PPAR-γ and GLUT4 are induced by a high glycemic load, leading to impaired insulin and a drastically increased risk of type 2 diabetes (Feil & Fraga, 2012). An example emphasising the risk of metabolic dysfunction is the Dutch Hunger Winter from 1994 to 1995, a severe famine caused by the German blockade. Individuals who were in utero during this period experienced irreversible epigenetic changes, including persistent hypomethylation at the IGF2 locus, which has been associated with metabolic dysfunction in adulthood (Heijmans et al., 2008).
Iron is essential for necessary processes such as dopaminergic neurotransmission, haemoglobin synthesis and myelination (Lozoff & Georgiff, 2006). Iron deficiency, more commonly known as anaemia or iron-deficiency anemia (IDA), affects an estimated 40% of children living in low-income countries, ultimately impairing their everyday functions such as memory and executive function (Lowkoski et al., 2010; WHO, 2021). Hippocampal DNA methylation is altered by prenatal iron deficiency, reducing synaptic plasticity genes BDNF and SLC17A7, directly increasing lifelong learning deficits (Tran et al., 2013). Th1 immune responses, DNA synthesis and wound healing are all supported by zinc (Prasad, 2013), with studies showing that a deficiency can lead to susceptibility to diarrhea and stunted growth (Wessels & Brown, 2012).
Vitamin D is associated with bone mineralisation and calcium homeostasis, and is responsible for the regulation of gene expression, immune function and neurodevelopment (Holick, 2017). Vitamin D deficiency (VDD), typically in early childhood, is still extremely common in places located at high latitudes and among populations that lack sun exposure (Holick, 2007). In more severe cases of VDD, the symptoms are manifested in diseases such as rickets, primarily characterised by bone deformities and slowed growth, while milder cases of VDD correlate to autoimmune disorders and respiratory infections (Hewison, 2012). Vitamin D receptors (VDR) are located and distributed in the brain, typically seen in the regions involved in social behaviour, memory and learning (Eyles et al., 2014). Animal studies in which vitamin D deficiency was induced have shown reductions in brain volume as well as altered cortical development and persistence (Elyes et al., 2014). Similarly, human studies suggest an association between maternal VDD and an increased risk of schizophrenia and autism spectrum disorder in offspring (Fernal et al., 2018).
Overall, keeping track of a child’s nutrition from an early age is critical as it sets a lifelong upward trajectory for their health (World Health Organization, 2018). As evidenced by various research on the developmental origins of disease and health, the lack of or excessive amounts of key micro- and macronutrients can affect many vital epigenetic modifications, such as DNA methylation in regulating gene expression (Barker, 2007). Moreover, key micronutrients, such as vitamin D and iron, are crucial for neural wiring and social-emotional learning (Georgiff, Ramel & Cusick, 2018). Therefore, the optimum intake of nutrients is extremely significant as it offers a wide range of benefits, rendering it an indispensable investment in the long term, ultimately serving as the foundation to prevent future illnesses.
The effects of macro- and micronutrient consumption on adolescent/pubertal development
Puberty is the transition from childhood to adulthood when the body undergoes physical, hormonal and sexual changes that lead to maturity and the ability to reproduce. Hormones are secreted, allowing physical development to occur and enabling sexual maturity. These hormones include gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), follicle-stimulating hormone (FSH) and sex hormones, such as estrogen and testosterone (Calcaterra et al., 2022). Puberty begins with hormonal signals from the hypothalamus and pituitary gland (Jordan, 2024). The hypothalamus starts producing GnRH and transports it to the pituitary gland, which releases FSH and LH. The hormones go to the reproductive organs, which release estrogen and testosterone. This signals puberty to start (Calcaterra et al., 2022). Most girls start puberty around 8-13 years of age, while most boys start from 10-15. Research has shown that the onset of puberty has progressively occurred at younger ages, with malnutrition identified as a primary contributing factor (Soliman et al., 2014).
Physical development during puberty is classified using the Tanner staging system. Stage 1 refers to the signs of puberty that occur before any observable physical changes, including the release of hormones, such as GnRH, to be transported to the pituitary gland. Stage 2 marks the beginning of physical development. Stage 3 and 4 mark increasingly noticeable changes in physical development, typically present in the form of a growth spurt. Stage 5 has occurred when the final adult height is reached. This is also the point when one has reached full sexual and physical maturity (Marcin, 2023).
Development can be affected by nutrition, which is needed to regulate hormones and aid functions during development (Jordan, 2024). Without proper nutrition, hormonal production can be low, resulting in delayed or advanced puberty (Soliman, De Sanctis & Elalaily, 2014). Various nutrients are required during puberty to support growth and development. Protein, for example, helps with muscle development, tissue growth and repair by providing amino acids for bodily functions. Calcium and vitamin D are needed to achieve the right bone mass and prevent osteoporosis later in life. Iron is needed for girls in particular during menstruation and for its production of haemoglobin, which carries oxygen to the blood. Zinc is needed for growth, development and the function of the immune system. Vitamin B is needed to regulate one’s metabolism, red blood cell production and neurotransmitter synthesis. Similarly, fat-soluble vitamins, such as vitamin A and E, help with hormonal regulation. Meanwhile, carbohydrates help fuel the body, especially the brain and muscles (Ferreira, 2024). Lastly, fats help absorb fat-soluble vitamins and are needed for hormone production and organ protection. Among all the nutrients, protein, calcium, vitamin D, carbohydrates and iron are the most crucial for reaching optimal height, repairing muscles and maintaining energy levels (Frey, 2024).
Poor nutrition choices, such as restrictive diets, can increase the risk of diseases in future generations through epigenetics. This happens through the production of methyl groups during DNA methylation, which can be disrupted. Methyl groups consist of one carbon atom and three hydrogen atoms, attaching to the DNA to act as a switch to turn genes on and off. Lacking certain nutrients can stop the production of methyl groups, leading to a change in gene expression (Bharaneetharan, Mohammad & Schneider, 2024). For example, lacking folate can impact brain development and function, which can affect one’s cognitive function in the long term (Mayo Clinic Staff, 2023). Nutrient deficiencies can also impact histone modifications, which can affect epigenetic enzymes and proteins, and microRNA activity, causing diseases and leading to irregular gene expression (Jordan, 2024).
Nutrition choices can also impact weight, which in turn impacts puberty. Overweight or obese boys are more likely to start puberty later, while overweight or obese girls are more likely to start puberty early. Being overweight during puberty can increase the chance of diseases, such as hyperandrogenemia in girls, where there is an excessive amount of androgens in the body. Similarly, being underweight can pose its own risks in pubertal development. In a study conducted with rats, overeating caused male rats to show excessive signs of puberty and female rats to release more hormones, causing hormonal imbalances. Undereating caused male rats to go through puberty later than normal and to develop insulin resistance, which could lead to diabetes. Lack of nutrients can also delay puberty in females (Soliman, De Sanctis, & Elalaily, 2014).
Malnutrition has multiple long-term effects such as nonlinear growth, which can cause catch-up growth later in life. Nonlinear growth refers to development in growth that is not consistent, meaning an individual may not reach optimal height at the expected age. Additionally, diseases such as cardiovascular disease, hypertension and metabolic syndrome are more common due to malnutrition during puberty, as well as muscle weakness when older. Lastly, malnutrition during puberty can increase the risk of cognitive disabilities (Kirolos et al., 2023).
To conclude, puberty is a key stage in healthy growth and development, and nutrition during puberty plays a major role in shaping physical, hormonal and long-term effects. Both malnutrition and excess nutrition during this stage can cause long-term changes to one’s development and to future generations through epigenetic changes, including DNA methylation, histone modification and microRNAs. By following balanced diets, adolescents can improve their development, prevent nutrient deficiencies, reduce the risk of diseases later in life and help prevent detrimental epigenetic effects on future generations.
The effects of accumulated epigenetic changes and adult diet on adult disease risk
Nutrition is a significant factor in epigenetics and therefore influences the expression of genes across different stages of life. Whether these epigenetic modifications are DNA methylation/acetylation, histone modifications or non-coding RNAs, they either encourage or discourage the expression of genes at different stages of life, requiring different genes to be expressed. Methylation can occur at both the histone level and on individual nucleotide bases, either increasing or decreasing gene expression. Acetylation and phosphorylation are two other epigenetic mechanisms, with the former increasing transcription rate and the latter playing a crucial role in DNA repair and mitosis. Non-coding RNAs can have a variety of roles, with one of the types, miRNA, repressing gene expression through complementary base pairing with mRNA to prevent translation of proteins (Dillinger, n.d.) However, some of these epigenetic modifications may persist and accumulate, manifesting as increased disease risk in adulthood (Sermon, 2017). This can be referred to as epigenetic drift, where gradual changes combined with early life imprints increase disease susceptibility. These changes may result from either nutrient deficiency or excess, with each carrying different consequences.
Maternal ingestion of certain compounds can influence foetal DNA methylation, which can result in persisting overactive or underactive states remaining in adulthood. This can be demonstrated through the Avy mouse model, whereby methylation of the agouti gene is manipulated to explore the effects of foetal DNA methylation on adult phenotype. Low methylation of the agouti gene leads to yellow fur and increases the risk of cancer, obesity and diabetes, whereas higher methylation (and lower expression) results in brown fur and a healthier metabolism (Dolinoy, 2008). The ingestion of restricted methyl donors, such as folate, decreases methylation in offspring, resulting in them presenting with a yellow coat and increased prevalence of the aforementioned ailments in adulthood (Chen & Zhang, 2011). Contrastingly, if the pregnant dame consumed genistein, the offspring demonstrated increased methylation at the agouti gene and a decreased risk of adult presentation of obesity (Dolinoy, 2008).
Nutrients during development can alter how DNA is packaged around histones, affecting whether genes are active or silent without changing the DNA code itself. Histone acetylation relaxes chromatin and hence increases transcription, whereas histone deacetylation compacts chromatin, silencing genes. Certain dietary components, such as butyrate and polyphenols, can inhibit histone deacetylases, causing the chromatin to maintain a more open structure, which alters metabolic gene expression (Choi & Friso, 2010). Once these modifications are established in early development, they can persist into adulthood, shaping long-term gene expression patterns linked to metabolism and disease risk (Waterland & Michels, 2007).
Non-coding RNAs such as microRNAs (miRNAs) can further influence metabolic gene expression. For instance, maternal high-fat diets cause a decrease in certain miRNAs that target proteins involved in gene regulation and epigenetics in rats, even when the offspring were raised with normal-fat diets, meaning that these altered metabolic states are carried into adulthood (Zhang et al., 2009).
Generally, foetal and childhood scarcity leads to adaptive changes to mitigate the effects of further nutrient deprivation, which may produce lasting changes in glucose-insulin metabolism (Hales & Barker, 2001). This is due to the developing foetus altering its gene expression to survive in the intrauterine environment, which, in times of scarce nutrition, can cause irreversible adaptations to metabolic setpoints. When the individual is later exposed to calorie-rich diets, the discrepancy between the internal system and the environmental conditions increases the risk for future disease (Chen & Zhang, 2011). Famine during the periconceptual period is linked to changes in methylation of genes related to growth and metabolic disease, with these consequences presenting in adulthood, most commonly as obesity or type 2 diabetes. The extent and type of these changes depend on the period when exposure to famine happens, but the effects are the most significant during the periconceptual period (Tobi et al., 2009).
Similarly, overnutrition during foetal development can increase adult disease risk through driving metabolic stress, reprogramming the epigenome to pro-inflammatory and pro-lipogenesis states. Zhang et al. (2019) found that high-fat diets, both during foetal development and after pup weaning, in rats can cause altered methylation of liver genes crucial for metabolic function. Even in the group where the high-fat consumption ceased after weaning, rats had higher blood sugar levels and poorer glucose control. Irs2, a gene crucial in helping the body respond to insulin, is hypermethylated in these groups, whereas Map2k4, a gene that can disrupt insulin signalling if overexpressed, is hypomethylated. These two factors lead to long-term glucose intolerance and insulin resistance, which are risk factors for type 2 diabetesAdult manifestations of type 2 diabetes and obesity are two diseases commonly associated with epigenetic modifications, as im. plied by the studies discussed above. This can further be visualised in Figure 1.
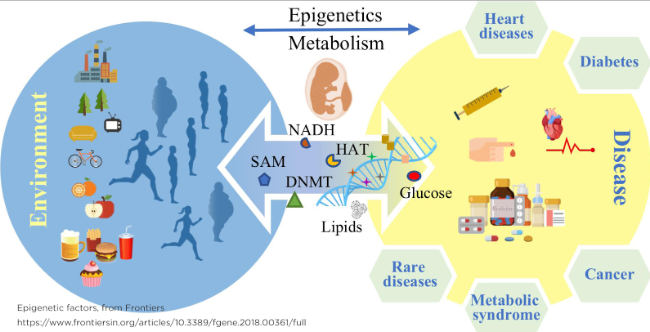
Figure 1: Diagram of epigenetic effects (Imhof, Tzika & Dreker, 2018).
Another group of diseases that can be attributed to epigenetics is cardiovascular diseases. As mentioned previously, famine during gestation and the periconceptual period can lead to offspring having altered methylation patterns that predispose them to growth and metabolic disorders. This, in turn, can lead to cardiovascular disease (CVD) (Gevaert et al., 2022). However, even adult nutrition can lead to epigenetic marks that increase the risk of cardiovascular disease. Although there does not exist a direct link between diet and epigenetic modifications that directly cause CVD, adult diet is associated with modifications that act as risk factors. For instance, Ma et al. (2020) found that diet could be linked to high levels of methylation of the gene that codes for a member of the fatty acid desaturase family, which is correlated with lower triglyceride concentrations. Nutrition’s influence on epigenetics can also be used to treat CVD with the Mediterranean diet, which has been shown to potentially improve endothelial function in patients with coronary heart disease (CHD) through increase in certain miRNAs (miR-188 and miR-25) and a decrease in others (miR181c, let-7e and miR-939) (Yubero-Serrano et al., 2020).
Beyond the individual risk for disease that epigenetic changes at the hands of nutrition can cause are the effects that can be passed on from the adult to the next generation. This implies that even the offspring of an individual exposed to adverse nutritional circumstances can carry these marks, despite never being exposed themselves. A natural experiment conducted by Bygren et al. (2014) found that if a paternal grandmother went through a significant change in nutritional availability, her granddaughters had a higher risk for cardiovascular mortality in adulthood, an association that was not found for maternal grandmothers or grandfathers. Although no direct epigenetic evidence was found to support these findings, the authors theorise that this trend could be attributed to X-linked miRNAs related to cardiovascular health, lending to the hypothesis that gametic epigenetic inheritance is the causative agent for the increased risk of cardiovascular mortality.
Conclusion
Nutrition is crucial in epigenetics as the regulation of hormones, development and gene expression can be impacted by macro- and micronutrients from birth until death. Nutrients are the foundation of health, supporting repair and function. Nutrients such as protein, iron, carbohydrates, calcium and vitamin D are among the most important nutrients to support brain function, cellular processes and the body’s systems. Nutrient deficiencies have consequences beyond increased hunger; health conditions increase in risk and epigenetic regulation can be damaged, changing the function of genes. The intake of nutrients is also important for epigenetic modifications. These include DNA methylation, histone modifications and microRNAs, which control how certain genes function. Malnutrition can impair these genes, leading to delays in growth and development. Since they are epigenetic modifications, the impairments may show up in following generations. This means that malnutrition during an individual’s lifetime not only affects them, but also has the potential to affect their children and grandchildren.
The effects of malnutrition can build over time. For example, malnutrition during pregnancy may create a rocky foundation for the foetus, creating issues before the child is born. Issues such as low birth weight or an increase in mortality rate for the child and mother can be a result of undernutrition during pregnancy. If the foetus is malnourished, they may face problems in adolescene with growth, development and increased risk of disease. Similarly, malnutrition during childhood can later affect the pubertal stage. It can delay puberty, disturb hormones, impair cognitive abilities and ultimately cause long-term health problems. Other effects, such as chronic diseases, can also appear later in life if nutrition remains insufficient into adulthood.
Ultimately, maintaining a nutritious diet should be seen as vital, not optional. The food we eat today will not only affect us as individuals, but also future generations to come.
Bibliography
Agustina, R., Rianda, D., Lasepa, W., Birahmatika, F.S., Stajic, V. & Mufida, R. (2023). Nutrient Intakes of Pregnant and Lactating Women in Indonesia and Malaysia: Systematic Review and meta-analysis. Frontiers in Nutrition, 10. doi:https://doi.org/10.3389/fnut.2023.1030343.
Ahmed, S.K. & Mohammed, R.A. (2025). Obesity: Prevalence, causes, consequences, management, Preventive Strategies and Future Research Directions. Metabolism Open, 27, p.100375. doi:https://doi.org/10.1016/j.metop.2025.100375.
Barker, D.J.P. (2007). The Origins of the Developmental Origins Theory. Journal of Internal Medicine, 261(5), pp.412–417. doi:https://doi.org/10.1111/j.1365-2796.2007.01809.x.
Benton, D. (2008). Micronutrient status, Cognition and Behavioral Problems in Childhood. European Journal of Nutrition, 47(S3), pp.38–50. doi:https://doi.org/10.1007/s00394-008-3004-9.
Berbel, P., Navarro, D. & Román, G.C. (2014). An Evo-Devo Approach to Thyroid Hormones in Cerebral and Cerebellar Cortical Development: Etiological Implications for Autism. Frontiers in Endocrinology, 5. doi:https://doi.org/10.3389/fendo.2014.00146.
Bharaneetharan, S., Mohammad, S. & Schneider, S. (2024). How different diets affect human epigenetics throughout generations. OxJournal. Available online: https://www.oxjournal.org/how-different-diets-affect-human-epigenetics-throughout-generations.
Black, R.E., Victora, C.G., Walker, S.P., Bhutta, Z.A., Christian, P., de Onis, M., Ezzati, M., Grantham-McGregor, S., Katz, J., Martorell, R. & Uauy, R. (2013). Maternal and Child Undernutrition and Overweight in low-income and middle-income Countries. The Lancet, 382(9890), pp.427–451. doi:https://doi.org/10.1016/S0140-6736(13)60937-X.
Blom, H.J., Shaw, G.M., den Heijer, M. & Finnell, R.H. (2006). Neural tube defects and folate: case far from closed. Nature Reviews Neuroscience, 7(9), pp.724–731. doi:https://doi.org/10.1038/nrn1986.
Bygren, L., Tinghög, P., Carstensen, J., Edvinsson, S., Kaati, G., Pembrey, M.E. & Sjöström, M. (2014). Change in paternal grandmothers´ early food supply influenced cardiovascular mortality of the female grandchildren. BMC Genetics, 15(1), p.12. doi:https://doi.org/10.1186/1471-2156-15-12.
Chen, M. & Zhang, L. (2011). Epigenetic mechanisms in developmental programming of adult disease. Drug Discovery Today, 16(23-24), pp.1007–1018. doi:https://doi.org/10.1016/j.drudis.2011.09.008.
Choi, S.-W. & Friso, S. (2010). Epigenetics: A New Bridge between Nutrition and Health. Advances in Nutrition, 1(1), pp.8–16. doi:https://doi.org/10.3945/an.110.1004.
Cleveland Clinic (n.d.). Puberty. Cleveland Clinic. Available online: https://my.clevelandclinic.org/health/body/puberty.
Compher, C et al., (2002). Choline and vitamin B12 deficiencies are interrelated in folate-replete long-term total parenteral nutrition patients. JPEN Journal of Parenteral and Enteral Nutrition, 26(1), pp. 57–62. doi:https://doi.org/10.1177/014860710202600157.
Cousins, R.J., Liuzzi, J.P. & Lichten, L.A. (2006). Mammalian Zinc Transport, Trafficking, and Signals. Journal of Biological Chemistry, 281(34), pp.24085–24089. doi:https://doi.org/10.1074/jbc.r600011200.
Crider, K.S., Yang, T.P., Berry, R.J. & Bailey, L.B. (2012). Folate and DNA Methylation: A Review of Molecular Mechanisms and the Evidence for Folate’s Role. Advances in Nutrition, 3(1), pp.21–38. doi:https://doi.org/10.3945/an.111.000992.
Crupi, R., Marino, A. & Cuzzocrea, S. (2013). n-3 Fatty Acids: Role in Neurogenesis and Neuroplasticity. Current Medicinal Chemistry, 20(24), pp.2953–2963. doi:https://doi.org/10.2174/09298673113209990140.
Dillinger, S. (n.d.). Complete Guide to Understanding Epigenetics. Active Motif. Available at: https://www.activemotif.com/epigenetics-101.
Dolinoy, D., Weidman, J. & Jirtle, R. (2007). Epigenetic Gene regulation: Linking Early Developmental Environment to Adult Disease. Reproductive Toxicology, 23(3), pp.297–307. doi:https://doi.org/10.1016/j.reprotox.2006.08.012.
Dolinoy, D.C. (2008). The Agouti Mouse model: an Epigenetic Biosensor for Nutritional and Environmental Alterations on the Fetal Epigenome. Nutrition Reviews, 66(1), pp.7–11. doi:https://doi.org/10.1111/j.1753-4887.2008.00056.x .
Dyall, S.C. (2015). Long-chain omega-3 Fatty Acids and the brain: a Review of the Independent and Shared Effects of EPA, DPA and DHA. Frontiers in aging neuroscience, 7(52), p.52. doi:https://doi.org/10.3389/fnagi.2015.00052.
Egger, G., Liang, G., Aparicio, A. & Jones, P.A. (2004). Epigenetics in human disease and prospects for epigenetic therapy. Nature, 429(6990), pp.457–463. doi:https://doi.org/10.1038/nature02625.
Emmanuel, M. & Bokor, B.R. (2022). Tanner stages. StatPearls [Internet]. StatPearls Publishing. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470280/.
Eyles, D.W., Burne, T.H.J. & McGrath, J.J. (2013). Vitamin D, Effects on Brain development, Adult Brain Function and the Links between Low Levels of Vitamin D and Neuropsychiatric Disease. Frontiers in Neuroendocrinology, 34(1), pp.47–64. doi:https://doi.org/10.1016/j.yfrne.2012.07.001.
Feil, R. & Fraga, M.F. (2012). Epigenetics and the environment: emerging patterns and implications. Nature Reviews Genetics, 13(2), pp.97–109. doi:https://doi.org/10.1038/nrg3142.
Fernell, E., Bejerot, S., Westerlund, J., Miniscalco, C., Simila, H., Eyles, D., Gillberg, C. & Humble, M.B. (2015). Autism spectrum disorder and low vitamin D at birth: a sibling control study. Molecular Autism, 6(1), p.3. doi:https://doi.org/10.1186/2040-2392-6-3.
Ferreira, M. (2024). Six essential nutrients: what they are and why you need them. Healthline. Available online: https://www.healthline.com/health/food-nutrition/six-essential-nutrients#protein.
Frey, L. (2024). Nutrition and puberty: the best way to feed your child’s growth. Available online: https://www.akronchildrens.org/inside/2024/03/27/nutrition-and-puberty-the-best-way-to-feed-your-childs-growth/.
Gavin, M., (2022) Water. KidsHealth. Available online: https://kidshealth.org/en/kids/water.html.
Georgieff, M.K. (2007). Nutrition and the Developing brain: Nutrient Priorities and Measurement. The American Journal of Clinical Nutrition, 85(2), pp.614S620S. doi:https://doi.org/10.1093/ajcn/85.2.614S.
Gevaert, A.B., Wood, N., Boen, J.R.A., Davos, C.H., Hansen, D., Hanssen, H., Krenning, G., Moholdt, T., Osto, E., Paneni, F., Pedretti, R.F.E., Plösch, T., Simonenko, M. & Bowen, T.S. (2022). Epigenetics in the primary and secondary prevention of cardiovascular disease: influence of exercise and nutrition. European Journal of Preventive Cardiology, 29(17), pp.2183–2199. doi:https://doi.org/10.1093/eurjpc/zwac179.
Gluckman, P.D., Hanson, M.A. & Buklijas, T. (2009). A Conceptual Framework for the Developmental Origins of Health and Disease. Journal of Developmental Origins of Health and Disease, 1(1), pp.6–18. doi:https://doi.org/10.1017/s2040174409990171.
Grunwald, T. (2023). Delayed puberty. KidsHealth. Available online: https://kidshealth.org/en/teens/delayed-puberty.html.
Hales, C.N. & Barker, D.J. (2001). The thrifty phenotype hypothesis. British Medical Bulletin, 60(1), pp.5–20. doi:https://doi.org/10.1093/bmb/60.1.5.
Heijmans, B.T., Tobi, E.W., Stein, A.D., Putter, H., Blauw, G.J., Susser, E.S., Slagboom, P.E. & Lumey, L.H. (2008). Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences, 105(44), pp.17046–17049. doi:https://doi.org/10.1073/pnas.0806560105.
Help Me Grow MN (n.d.) What is cognitive development. Help Me Grown MN. Available online: https://helpmegrowmn.org/HMG/HelpfulRes/Articles/WhatCognitiveDev/index.html.
Hernandez, T.L. & Rozance, P.J. (2022). Re-examination of the Estimated Average Requirement for Carbohydrate Intake during pregnancy: Addition of Placental Glucose Consumption. The American Journal of Clinical Nutrition, 117(2). doi:https://doi.org/10.1016/j.ajcnut.2022.09.005.
Herring, C.M., Bazer, F.W., Johnson, G.A. & Wu, G. (2018). Impacts of Maternal Dietary Protein Intake on Fetal survival, growth, and Development. Experimental Biology and Medicine, 243(6), pp.525–533. doi:https://doi.org/10.1177/1535370218758275.
Hewison, M. (2011). Vitamin D and Immune function: an Overview. Proceedings of the Nutrition Society, 71(1), pp.50–61. doi:https://doi.org/10.1017/s0029665111001650.
Hibbeln, J.R., Davis, J.M., Steer, C., Emmett, P., Rogers, I., Williams, C. & Golding, J. (2007). Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. The Lancet, 369(9561), pp.578–585. doi:https://doi.org/10.1016/s0140-6736(07)60277-3.
Holick, M.F. (2007). Vitamin D Deficiency. The New England journal of medicine, 357(3), pp.266–81. doi:https://doi.org/10.1056/NEJMra070553.
Imhof, A., Tzika, E. & Dreker, T. (2018). Environmental Factors Affect Epigenetics and Metabolism and Control Disease Predisposition in Later Life stages. [Online Image] Frontiers. Available online: https://www.frontiersin.org/journals/genetics/articles/10.3389/fgene.2018.00361/full.
Innis, S.M. (2008). Dietary Omega 3 Fatty Acids and the Developing Brain. Brain research, 1237, pp.35–43. doi:https://doi.org/10.1016/j.brainres.2008.08.078.
Jeffery, L.E., Burke, F., Mura, M., Zheng, Y., Qureshi, O.S., Hewison, M., Walker, L.S.K., Lammas, D.A., Raza, K. & Sansom, D.M. (2009). 1,25-Dihydroxyvitamin D3 and IL-2 Combine to Inhibit T Cell Production of Inflammatory Cytokines and Promote Development of Regulatory T Cells Expressing CTLA-4 and FoxP3. The Journal of Immunology, 183(9), pp.5458–5467. doi:https://doi.org/10.4049/jimmunol.0803217.
Jordan, J (2024). The Role of Nutrition in Puberty and Adolescent Development. Reproductive System & Sexual Disorders: Current Research, 13(4), p. 437. Available online: https://www.longdom.org/open-access/the-role-of-nutrition-in-puberty-and-adolescent-development-110301.html.
Khammarnia, M., Ansari-Moghaddam, A., Govahi Kakhki, F., Truman, C. & Bagher Barahouei, F. (2024). Maternal macronutrient and energy intake during pregnancy: a systematic review and meta-analysis. BMC Public Health, 24(1). doi:https://doi.org/10.1186/s12889-024-17862-x.
Kirolos, A. & Abera, M. et al, (2023). The effect of childhood nutrition on growth and development. The Lancet Child & Adolescent Health, 7(5), pp. 321–330. doi:https://doi.org/10.1016/S2352-4642(23)00339-5.
Kizirian, N.V., Kong, Y., Muirhead, R., Brodie, S., Garnett, S.P., Petocz, P., Sim, K.A., Celermajer, D.S., Louie, J.C., Markovic, T.P., Ross, G.P., Ward, L.C., Brand-Miller, J.C. & Skilton, M.R. (2016). Effects of a Low–glycemic Index Diet during Pregnancy on Offspring growth, Body composition, and Vascular health: a Pilot Randomized Controlled Trial. The American Journal of Clinical Nutrition, 103(4), pp.1073–1082. doi:https://doi.org/10.3945/ajcn.115.123695.
Lillycrop, K.A., Phillips, E.S., Jackson, A.A., Hanson, M.A. & Burdge, G.C. (2005). Dietary Protein Restriction of Pregnant Rats Induces and Folic Acid Supplementation Prevents Epigenetic Modification of Hepatic Gene Expression in the Offspring. The Journal of Nutrition, 135(6), pp.1382–1386. doi:https://doi.org/10.1093/jn/135.6.1382.
Lozoff, B. & Georgieff, M.K. (2006). Iron Deficiency and Brain Development. Seminars in Pediatric Neurology, 13(3), pp.158–165. doi:https://doi.org/10.1016/j.spen.2006.08.004.
Ludwig, D.S. & Ebbeling, C.B. (2018). The Carbohydrate-Insulin Model of Obesity. JAMA Internal Medicine, 178(8), p.1098. doi:https://doi.org/10.1001/jamainternmed.2018.2933.
Lukowski, A.F., Koss, M., Burden, M.J., Jonides, J., Nelson, C.A., Kaciroti, N., Jimenez, E. & Lozoff, B. (2010). Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutritional Neuroscience, 13(2), pp.54–70. doi:https://doi.org/10.1179/147683010×12611460763689.
Ma, J., Rebholz, C.M., Kim V.E. Braun, Reynolds, L.M., Aslibekyan, S., Xia, R., Biligowda, N.G., Huan, T., Liu, C., Mendelson, M.M., Roby Joehanes, Hu, E.A., Vitolins, M.Z., Wood, A.C., Lohman, K., Ochoa-Rosales, C., Joyce van Meurs, Uitterlinden, A., Liu, Y. & Elhadad, M.A. (2020). Whole Blood DNA Methylation Signatures of Diet Are Associated with Cardiovascular Disease Risk Factors and All-Cause Mortality. Circulation. Genomic and precision medicine, 13(4). doi:https://doi.org/10.1161/circgen.119.002766.
Marcin, A., (2023). Stages of puberty: a guide for males and females. Healthline. Available online: https://www.healthline.com/health/parenting/stages-of-puberty#summary.
Martínez, J.A., Milagro, F.I., Claycombe, K.J. & Schalinske, K.L. (2014). Epigenetics in Adipose Tissue, Obesity, Weight Loss, and Diabetes. Advances in Nutrition, 5(1), pp.71–81. doi:https://doi.org/10.3945/an.113.004705.
Mayo Clinic Staff (2023). Folate (folic acid). Mayo Clinic. Available online: https://www.mayoclinic.org/drugs-supplements-folate/art-20364625.
McCann, J.C. & Ames, B.N. (2005). Is Docosahexaenoic acid, an n−3 long-chain Polyunsaturated Fatty acid, Required for Development of Normal Brain function? an Overview of Evidence from Cognitive and Behavioral Tests in Humans and Animals. The American Journal of Clinical Nutrition, 82(2), pp.281–295. doi:https://doi.org/10.1093/ajcn/82.2.281.
Michigan State University (2010). High-fat diet during puberty linked to breast cancer risk later in life. ecancer. Available online: https://ecancer.org/en/news/1187-high-fat-diet-during-puberty-linked-to-breast-cancer-risk-later-in-life.
Mousa, A., Naqash, A. & Lim, S. (2019). Macronutrient and Micronutrient Intake during Pregnancy: an Overview of Recent Evidence. Nutrients, 11(2), p.443. doi:https://doi.org/10.3390/nu11020443.
Niculescu, M.D. & Zeisel, S.H. (2002). Diet, Methyl Donors and DNA Methylation: Interactions between Dietary Folate, Methionine and Choline. The Journal of Nutrition, 132(8), pp.2333S2335S. doi:https://doi.org/10.1093/jn/132.8.2333s.
Padmanabhan, N., Jia, D., Geary-Joo, C., Wu, X., Ferguson-Smith, Anne C., Fung, E., Bieda, Mark C., Snyder, Floyd F., Gravel, Roy A., Cross, James C. & Watson, Erica D. (2013). Mutation in Folate Metabolism Causes Epigenetic Instability and Transgenerational Effects on Development. Cell, 155(1), pp.81–93. doi:https://doi.org/10.1016/j.cell.2013.09.002.
Painter, R., Osmond, C., Gluckman, P., Hanson, M., Phillips, D. & Roseboom, T. (2008). Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG: An International Journal of Obstetrics & Gynaecology, 115(10), pp.1243–1249. doi:https://doi.org/10.1111/j.1471-0528.2008.01822.x.
Prado, E.L. & Dewey, K.G. (2014). Nutrition and Brain Development in Early Life. Nutrition Reviews, 72(4), pp.267–284. doi:https://doi.org/10.1111/nure.12102.
Prasad, A.S. (2013). Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Advances in Nutrition, 4(2), pp.176–190. doi:https://doi.org/10.3945/an.112.003210.
Reynolds, E.H. (2014). The Neurology of Folic Acid Deficiency. Handbook of Clinical Neurology, 120, pp.927–943. doi:https://doi.org/10.1016/B978-0-7020-4087-0.00061-9.
Sermon, K. (2017). Preimplantation Genetic Screening. OBM Genetics, 1(4), pp.1–1. doi:https://doi.org/10.21926/obm.genet.1704008.
Socha, M.W., Flis, W. & Wartęga, M. (2024). Epigenetic Genome Modifications during Pregnancy: the Impact of Essential Nutritional Supplements on DNA Methylation. Nutrients, 16(5), p.678. doi:https://doi.org/10.3390/nu16050678.
Soliman, A., De Sanctis, V. & Elalaily, R. (2014) Nutrition and pubertal development. Indian Journal of Endocrinology and Metabolism, 18 (1), pp.39–47. doi:https://doi.org/10.4103/2230-8210.145073.
Stephenson, J., Heslehurst, N., Hall, J., Schoenaker, D.A.J.M., Hutchinson, J., Cade, J.E., Poston, L., Barrett, G., Crozier, S.R., Barker, M., Kumaran, K., Yajnik, C.S., Baird, J. & Mishra, G.D. (2018). Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. The Lancet, 391(10132), pp.1830–1841.
Thomas, M. & Weisman, S.M. (2006). Calcium Supplementation during Pregnancy and lactation: Effects on the Mother and the Fetus. American Journal of Obstetrics and Gynecology, 194(4), pp.937–945. doi:https://doi.org/10.1016/j.ajog.2005.05.032.
Tobi, E.W., Goeman, J.J., Monajemi, R., Gu, H., Putter, H., Zhang, Y., Slieker, R.C., Stok, A.P., Thijssen, P.E., Müller, F., van Zwet, E.W., Bock, C., Meissner, A., Lumey, L.H., Eline Slagboom, P. & Heijmans, B.T. (2014). DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nature Communications, 5(1), p.5592. doi:https://doi.org/10.1038/ncomms6592.
Tobi, E.W., Lumey, L.H., Talens, R.P., Kremer, D., Putter, H., Stein, A.D., Slagboom, P.E. & Heijmans, B.T. (2009). DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Human Molecular Genetics, 18(21), pp.4046–4053. doi:https://doi.org/10.1093/hmg/ddp353.
Tran, P.V., Fretham, S.J.B., Wobken, J., Miller, B.S. & Georgieff, M.K. (2012). Gestational-neonatal iron deficiency suppresses and iron treatment reactivates IGF signaling in developing rat hippocampus. American Journal of Physiology-Endocrinology and Metabolism, 302(3), pp.316–324. doi:https://doi.org/10.1152/ajpendo.00369.2011.
Viner, R., Allen, N., & Patton, G. (2016). Puberty, Developmental Processes, and Health Interventions. Child and Adolescent Health and Development. Available online: https://www.ncbi.nlm.nih.gov/books/NBK525269/ .
Vucetic, Z., Kimmel, J., Totoki, K., Hollenbeck, E. & Reyes, T.M. (2010). Maternal High-Fat Diet Alters Mthylation and Gene Expression of Dopamine and Opioid-Related Genes. Endocrinology, 151(10), pp.4756–4764. doi:https://doi.org/10.1210/en.2010-0505.
Waterland, R.A. (2006). Epigenetic Mechanisms and Gastrointestinal Development. The Journal of Pediatrics, 149(5), pp.137–142. doi:https://doi.org/10.1016/j.jpeds.2006.06.064.
Waterland, R.A. & Jirtle, R.L. (2003). Transposable Elements: Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Molecular and Cellular Biology, 23(15), pp.5293–5300. doi:https://doi.org/10.1128/mcb.23.15.5293-5300.2003.
Waterland, R.A. & Michels, K.B. (2007). Epigenetic Epidemiology of the Developmental Origins Hypothesis. Annual Review of Nutrition, 27(1), pp.363–388. doi:https://doi.org/10.1146/annurev.nutr.27.061406.093705.
Wessells, K.R. & Brown, K.H. (2012). Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLoS ONE, 7(11), p.e50568. doi:https://doi.org/10.1371/journal.pone.0050568.
WHO (2025). Anaemia in Women and Children. World Health Organization. Available online: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children.
World Health Organization (2007). Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination : a Guide for Programme Managers. apps.who.int. Available online: https://apps.who.int/iris/handle/10665/43781.
World Health Organization (2020). Guideline: Protein intake for adults and children. World Health Organization. Available online: https://www.who.int/publications/i/item/9789241547693.
Xue, L., Chen, X., Sun, J., Fan, M., Qian, H., Li, Y. & Wang, L. (2024). Maternal Dietary Carbohydrate and Pregnancy Outcomes: Quality over Quantity. Nutrients, 16(14), pp.2269–2269. doi:https://doi.org/10.3390/nu16142269.
Yang, J., Chang, Q., Tian, X., Zhang, B., Zeng, L., Yan, H., Dang, S. & Li, Y.-H. (2022). Dietary Protein Intake during Pregnancy and Birth Weight among Chinese Pregnant Women with Low Intake of Protein. Nutrition & Metabolism, 19(1). doi:https://doi.org/10.1186/s12986-022-00678-0.
Yubero-Serrano, E.M., Fernandez-Gandara, C., Garcia-Rios, A., Rangel-Zuñiga, O.A., Gutierrez-Mariscal, F.M., Torres-Peña, J.D., Marin, C., Lopez-Moreno, J., Castaño, J.P., Delgado-Lista, J., Ordovas, J.M., Perez-Martinez, P. & Lopez-Miranda, J. (2020). Mediterranean diet and endothelial function in patients with coronary heart disease: An analysis of the CORDIOPREV randomized controlled trial. PLoS Medicine, 17(9). doi:https://doi.org/10.1371/journal.pmed.1003282.
Zhang, J., Zhang, F., Didelot, X., Bruce, K.D., Cagampang, F.R., Vatish, M., Hanson, M., Lehnert, H., Ceriello, A. & Byrne, C.D. (2009). Maternal high fat diet during pregnancy and lactation alters hepatic expression of insulin like growth factor-2 and key microRNAs in the adult offspring. BMC Genomics, 10(1), p.478. doi:https://doi.org/10.1186/1471-2164-10-478.
Zhang, Q., Xiao, X., Zheng, J., Li, M., Yu, M., Ping, F., Wang, T. & Wang, X. (2019). A Maternal High-Fat Diet Induces DNA Methylation Changes That Contribute to Glucose Intolerance in Offspring. Frontiers in Endocrinology, 10. doi:https://doi.org/10.3389/fendo.2019.00871.
Zimmermann, M.B. (2009). Iodine Deficiency. Endocrine Reviews, 30(4), pp.376–408. doi:https://doi.org/10.1210/er.2009-0011.




