Abstract
In a world afflicted by devastating events, ranging from wars and unimaginable violence to tragic accidents and natural disasters, an estimated 70% of the human population will experience a potentially traumatising incident during their lifetime, and nearly 5.6% of them will develop post-traumatic stress disorder (PTSD), one of the most distressing mental illnesses worldwide. This paper reviews the existing literature on the connection between PTSD and epigenetic inheritance, which provides valuable insights into how trauma can leave biological, non-genetic imprints with consequences for future generations. This article therefore explores the epigenetic dimension of post-traumatic stress disorder and underscores the critical assertion that the effects of traumatic experiences do not end with those directly exposed. Accordingly, its aim is to raise awareness of the epigenetic heritability of PTSD, while highlighting its implications for today’s society and public health system. Key findings include the central role of DNA methylation in the regulation of stress response, the major significance of parental traumatic exposure on offspring mental health and PTSD predisposition, the increasingly recognised role of noncoding RNAs in epigenetic inheritance, and the potential reversibility of the disorder’s symptoms through environmental enrichment therapy. Ultimately, this review renders the understanding of the epigenetics in trauma and PTSD a pressing necessity for building a society rooted in mental wellbeing.
1. INTRODUCTION
Epigenetics is a growing field of study which explains the mechanisms by which gene expression is modulated without alterations to the primary DNA sequence, thereby offering a crucial understanding of the interplay between environmental factors and the genome. More specifically, epigenetics refers to direct modifications in DNA regulation that do not affect the primary sequence of DNA or genetic code (Howie et al., 2019). Epigenetic modifications involve DNA methylation, histone modification and non-coding RNA regulation which orchestrate gene expression patterns (Aykac & Kalkan, 2021). Epigenetic modifications can influence chromatin state and thus the accessibility of transcriptional machinery to regulatory regions such as gene promoters, resulting in either targeted or genome-wide changes in gene expression (Matosin et al., 2017). These processes are vital for normal development and cellular differentiation, but aberrant epigenetic patterns have been implicated in various diseases, including cancer, neurodevelopmental disorders and psychiatric conditions (Zovkic et al., 2013). The dynamism of epigenetic marks allows for adaptive responses to environmental cues, but also raises the possibility of maladaptive programming, especially in the context of early-life stress and trauma (Matosin et al., 2017).
Over the past several decades, the prevalence of mental health disorders has been steadily increasing worldwide (Foy, 2024). Among mental health diseases, post-traumatic stress disorder (PTSD) is a debilitating psychiatric condition that arises following exposure to a traumatic event, characterised by a constellation of psychological, physiological and emotional disturbances. The traumatic events that can trigger PTSD are diverse, including violent combat exposure, natural disasters, serious accidents, terrorist attacks, physical or sexual assault, and other life-threatening situations (Addissouky et al., 2025). Beyond the individual, the ramifications of PTSD extend to families and future generations, suggesting that the effects of trauma may not be confined to those directly exposed (Sirikantraporn & Green, 2016). PTSD is characterised by intrusive thoughts, nightmares, avoidance behaviours, negative alterations in cognition and mood, and heightened arousal and reactivity (Addissouky et al., 2025). These symptoms of PTSD can significantly impair an individual’s quality of life, interpersonal relationships and occupational functioning, thereby imposing a substantial burden on healthcare systems and society as a whole (Aykac & Kalkan, 2021). Epigenetic changes directly affect the severity of PTSD by modifying genes involved in the body’s stress response. They alter neural pathways and emotional regulation. These alterations can lead to long-lasting effects on how individuals process and cope with traumatic experiences (Addissouky et al., 2025).
Epigenetic inheritance represents a paradigm shift in our understanding of heredity, moving beyond the classical gene-centric view to encompass the transmission of information across generations via mechanisms independent of the DNA sequence itself (Yehuda & Lehrner, 2018). These epigenetic marks, acquired in response to environmental exposures or experiences, can potentially reprogram the germline, leading to heritable changes in gene expression and physiological function across multiple generations (Jawaid et al., 2018). Namely, transgenerational epigenetic inheritance refers to the transmission of epigenetic modifications across multiple generations, beyond those directly exposed to the original environmental factor (Moore, 2015). Intergenerational epigenetic inheritance, on the other hand, refers to the transmission of epigenetic information from parent to direct offspring, typically the first (F1) or sometimes the second (F2) generation (Sustainability Directory, 2025). The implications of transgenerational and intergenerational epigenetic inheritance are particularly relevant in the context of trauma, where the experiences of one generation can have profound and lasting effects on subsequent generations. More provocatively speaking, the experience of trauma is somehow “passed” from one generation to the next, possibly through epigenetic mechanisms (Yehuda & Lehrner, 2018). Psychological trauma that stems from extremely distressing events has long been recognised for its immediate and long-term consequences for mental and physical health (Afifah Ridhuan et al., 2021). Accumulating evidence suggests that the effects of trauma can extend beyond the directly-exposed individuals, impacting their descendants through mechanisms that are only beginning to be elucidated (Sirikantraporn & Green, 2016). This intergenerational transmission of trauma has traditionally been attributed to psychosocial factors, such as impaired parenting styles, maladaptive coping mechanisms and the perpetuation of traumatic narratives within families. However, emerging research suggests that biological mechanisms, particularly epigenetic modifications, may also play a critical role in mediating the inheritance of trauma-related phenotypes (Jawaid et al., 2018).
In this article, we review the growing body of evidence supporting the intergenerational and transgenerational epigenetic inheritance of trauma, particularly in relation to PTSD. In addition, we identify key biological mechanisms, animal and human case studies, and the limitations and challenges of scientific validation regarding this phenomenon. Finally, we highlight the need for trauma-informed healthcare systems and further interdisciplinary research into intergenerational mental health.
2. EPIGENETIC MECHANISMS UNDERLYING PTSD AND THE INHERITANCE OF TRAUMA ACROSS GENERATIONS
Post-traumatic stress disorder is a psychiatric disease with both a genetic and an epigenetic component (Howie et al., 2019). Although counterintuitive, as PTSD is relevant to personal traumatic experiences, the heritability of the risk of the disorder is gradually being established, with already significant findings in rodent studies and emerging evidence in humans. These point to epigenetic mechanisms that modulate the heritable component of PTSD, rendering the disorder a matter of both intergenerational and transgenerational epigenetic inheritance. Current studies are investigating the impact of parental trauma on offspring vulnerability or resilience to PTSD. This involves studying concepts such as epigenetic reprogramming, genomic imprinting and epigenetic mechanisms, including DNA methylation, histone modifications and noncoding RNAs, in order to determine their influence on the mental health of individuals.
In this part of the review, we will discuss how these mechanisms are associated with PTSD and how parental experiences may influence offspring susceptibility to the disorder. We will also address plausible assumptions on how trauma – as well as PTSD – affects the germline, is passed down to future generations and shapes individuals’ stress response by affecting their neurodevelopment. We also outline hallmark studies conducted on both rodents and humans in this area of research, highlighting the potentially enduring effects of trauma and PTSD across multiple generations.
2.1 EPIGENETIC MECHANISMS RELEVANT TO PTSD
Recent studies in both rodents and humans have implicated epigenetic modifications in the development of post-traumatic stress disorder (Zovkic et al., 2013). Epigenetic factors may be integral to PTSD predisposition, symptom severity and progression, potentially constituting promising targets for therapeutic intervention (Cao-Lei et al., 2022; Golubeva et al., 2024).
PTSD is induced by the exposure to traumatic experiences (Du et al., 2022; Howie et al., 2019). This makes it fundamentally rooted in environmental factors (Golubeva et al., 2024) and sets it apart from other psychiatric conditions, such as bipolar disorder, by emphasising the interplay between nature and nurture (Howie et al., 2019). This paper begins by outlining the three most studied epigenetic mechanisms which may be underlying this interaction in PTSD development: DNA methylation, histone modifications and noncoding RNA action.
2.1.1 DNA METHYLATION
It is suggested that DNA methylation influences fundamental processes relevant to the onset and persistence of PTSD (Bhattacharya et al., 2019; Zovkic et al., 2013). DNA methylation in PTSD development has been profoundly studied through epigenome-wide association studies (EWAS) (Golubeva et al., 2024), which are investigations aiming to identify disease-specific biomarkers (Tanić, 2020). DNA methylation refers to the enzymatic process by which a methyl group (CH₃) is added to the fifth position of a cytosine residue (5-methylcytosine, 5mC), and mostly to CpG dinucleotides, using enzymes known as DNA methyltransferases (DNMTs) (Lacal & Ventura, 2018). This whole process, concerning the addition and removal of epigenetic markers, requires epigenetic regulatory proteins, known as readers, writers and erasers (Chou et al., 2024).
DNA methylation was initially only associated with gene silencing, which takes place either by preventing transcription factors from binding to DNA, or by recruiting methyl-CpG binding proteins (MBPs) and related repressive chromatin remodelling components. However, recent studies have shown that DNA methylation can also facilitate gene activation if present at the promoter and coding region of actively transcribed genes. This suggests the heterogeneity of this mechanism (Jawaid et al., 2018; Zovkic & Sweatt, 2013).
There are two types of DNA methylation: de novo methylation, which refers to the addition of methyl groups to previously unmethylated cytosine bases and is primarily carried out by DNMT3A and DNMT3B to establish new DNA methylation patterns; and maintenance DNA methylation that preserves already existing methylation profiles during DNA replication by methylating cytosines on newly synthesised strands opposite methylated parental strands, a process predominantly mediated by DNMT1 (Lacal & Ventura, 2018; Zovkic et al., 2013). The demethylation of CpGs is initiated by the oxidation of 5mC to 5-hydroxymethylcytosine (5hmC), which is catalysed by ten-eleven translocation (TET) proteins. This process is known as DNA hydroxymethylation. The relatively high concentration and stability of 5hmC in the rodent and human brain suggests that this modification is vital to neuronal activity and brain function (Jawaid et al., 2018; Zovkic et al., 2013).
Amongst other DNA modifications, 5mC and 5hmC are the two most prevalent methylation-related alterations that dominate neuronal activity (Zovkic et al., 2013). Their dysregulation is associated with impaired cognitive processes involved in PTSD, including memory and fear extinction (van Marle, 2015; Wicking et al., 2016), which refers to the process of learning that a once-threatening cue is no longer dangerous (Hefner et al., 2008). Several studies have identified modifications in methylation states in both PTSD patients and their offspring, such as in the case of Holocaust survivors and their progeny (Yehuda & Lehrner, 2018). These alterations especially concern the glucocorticoid receptor (GR), a protein to which cortisol and other glucocorticoids (GCs) bind to exert their effects. GR, which is encoded by the NR3C1 gene (nuclear receptor subfamily 3, group C, member 1), is integral to the regulation of the hypothalamic-pituitary-adrenal (HPA) axis (Bhattacharya et al., 2019). The methylation of HPA axis genes involved in PTSD will be addressed further below.
The methylation-related alterations induced by traumatic experiences suggest a potential role for DNA methylation in the long-term impact of traumatic stress, contributing to the development of acute stress disorder (ASD) and PTSD (Jawaid et al., 2018). Furthermore, this epigenetic mechanism is also involved in the regulation of fear memory. After a traumatic event, there is elevated activity of DNA methyltransferases in the brain, which contributes to the regulation of genes involved in memory consolidation, including fear memories, thereby influencing their formation and storage (Day & Sweatt, 2011; Maddox et al., 2013; Zovkic & Sweatt, 2013). This may also underscore the role of DNA methylation in the enduring effects of trauma in the brain (Jawaid et al., 2018).
Finally, DNA methylation has also been implicated in the regulation of the BDNF gene (Zovkic & Sweatt, 2013), which encodes for brain-derived neurotrophic factor, a protein involved in cognitive processes such as fear learning and memory (Antal et al., 2010). Studies have shown that the expression of BDNF can be modified by early-life traumatic experiences (e.g. Jeon et al., 2012; Jin et al., 2013; Roth et al., 2009). Notably, such alterations may persist even in the absence of the original threat, suggesting a role for BDNF dysregulation in the persistence of pathological fear, a hallmark of PTSD (Zovkic & Sweatt, 2013). A key study by Roth et al. (2009) demonstrated the relationship between DNA methylation and BDNF expression using a rodent model. Offspring exposed to an abusive dam during the early postnatal period exhibited increased methylation and decreased expression of BDNF in the prefrontal cortex in adulthood. This research showed that early maternal maltreatment led to lasting epigenetic changes in the BDNF gene, potentially predisposing the offspring to PTSD-like behaviour. Overall, the study highlights the significant role of DNA methylation in cognition, particularly in the regulation of fear memory, which is central to the pathophysiology of PTSD (Zovkic & Sweatt, 2013).
2.1.2 PTSD, DNA METHYLATION AND THE HPA AXIS
Along with other differentially methylated genes related to neurodevelopment, immune function, metabolism and cellular processes (Golubeva et al., 2024), DNA methylation has also been associated with the dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and altered stress response, especially in terms of cortisol levels and the transcription of glucocorticoid receptors (GR), which is seriously implicated in the etiology and pathophysiology of PTSD (Bhattacharya et al., 2019; Zovkic et al., 2013). As stress hormones are integral to stress response, and cortisol is the dominant stress hormone in humans, preparing the body to react to environmental stimuli and stressors (Bhattacharya et al., 2019), its dysregulation can have detrimental consequences for mental health, increasing the risk of developing PTSD. The HPA axis refers to a stress response pathway that develops in early prenatal stages and is highly sensitive to environmental stimuli (Chou et al., 2024). It is also related to immune system regulation and metabolism, which are also affected in PTSD (Golubeva et al., 2024). Stressful encounters prompt an endocrinological circuit in the HPA axis to release corticotropin-releasing hormone (CRH) from the hypothalamus into the pituitary gland, which in turn stimulates the release of adrenocorticotropin hormone (ACTH). This hormone then triggers the release of glucocorticoids (i.e., cortisol in humans) from the adrenal glands, helping mobilise energy stores, regulate heart rate and modulate the immune system in response to the stimuli. Cortisol inhibits the release of ACTH and consequently prevents its own release, thereby creating a negative feedback loop, so as to restore homeostasis (Bhattacharya et al., 2019; Jawaid et al., 2018; Sapolsky et al., 1985; Zovkic et al., 2013).
Traumatic stress alters the integrity of the HPA axis, influencing stress resilience, memory encoding, and social and emotional responses (Jawaid et al., 2018). In some individuals functional changes in the axis have been observed in the aftermath of a traumatic event, which could potentially constitute a risk factor for the development of PTSD (Golubeva et al., 2024). In fact, epigenetic alterations in the stress neurocircuitry that lead to its dysregulation, have been described as a pre-existing susceptibility that heightens the risk of PTSD development (Rodgers & Bale, 2015). PTSD patients generally exhibit alterations in the HPA axis, low cortisol levels, elevated negative feedback and increased levels of CRH. These alterations are also combined with modified responses in brain regions strongly associated with the HPA axis, specifically in the amygdala and the hippocampus that are also involved in cognition. This indicates that a blunted GC stress response may be connected to PTSD risk (Jawaid et al., 2018; Zovkic et al. 2013).
Characteristic examples indicating the relation of DNA methylation to stress response and risk of PTSD development are the studies conducted on rats that received high or low maternal care (e.g., Weaver et al., 2004) and the studies on Holocaust survivors and their children (e.g., Bierer et al., 2020; Bowers & Yehuda, 2015; Yehuda et al., 2016). Rat progeny of high maternal care mothers exhibited lower methylation of the NR3C1 promoter (i.e., the DNA region that controls the expression of the NR3C1 gene that encodes for GR), leading to increased GR expression and greater feedback sensitivity. Conversely, low maternal care was associated with increased NR3C1 methylation, reduced GR expression and blunted feedback regulation of the HPA axis. Interestingly, these findings parallel HPA axis activity in PTSD patients, who often exhibit hypocortisolism and elevated GR sensitivity (Lawrence & Scofield, 2024). Similar patterns have been reported in children of Holocaust survivors, who showed low cortisol levels, while rating their mothers as overprotective (Yehuda & Bierer, 2009). The prevailing hypothesis suggests that elevated GR sensitivity induces elevated negative feedback within the HPA axis, causing the system to suppress cortisol production, even in response to small amounts of the hormone, which could potentially account for prolonged hypocortisolism (Lawrence & Scofield, 2024).
Aberrant expression of FK506 binding protein 5 (FKBP5), which is linked to HPA axis dysregulation, has also been shown to be modulated by DNA methylation. FKBP5 is a negative regulator of GR activity (Zovkic et al., 2013), lowering cortisol affinity when it is bound to GR (Bhattacharya et al., 2019), thereby regulating GR sensitivity to glucocorticoids (Golubeva et al., 2024). In the case of Holocaust survivors, FKBP5 methylation in parents and their offspring were found to be intergenerationally associated. However, Holocaust-exposed individuals showed higher methylation in this site compared to their respective control groups, whereas their offspring exhibited lower methylation compared to controls (Yehuda & Lehrner, 2018).
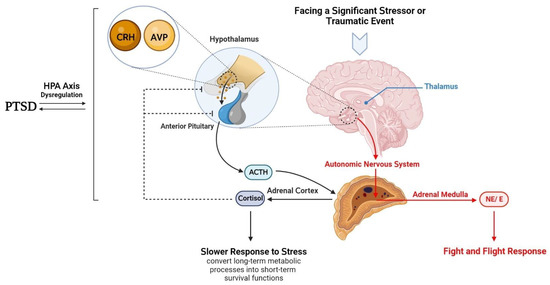
Figure 1: Normal function of the hypothalamic-pituitary-adrenal (HPA) axis after exposure to a stressor. Dysfunction of the HPA axis, for instance due to aberrant DNA methylation, has been associated with mental disorders, including PTSD. Taken from https://www.mdpi.com/2076-3425/13/7/1010.
2.1.3 HISTONE MODIFICATIONS
Chromatin regulation, which is primarily mediated by histone modifications, is also involved in PTSD. Histone proteins (H2A, H2B, H3, H4) contribute to the organisation of DNA into structured units, called nucleosomes, which are the building blocks of chromatin. They ensure that DNA is wrapped around an octamer of histones, in a compact structure, and they are therefore vital to gene regulation, as they determine DNA accessibility by either creating an open (euchromatin) or a compressed (heterochromatin) chromatin structure (Chou et al., 2024; Howie et al., 2019; Zovkic et al., 2013). Histones also contain protruding tails that are prone to chemical modifications, known as histone post-translational modifications (HPTMs). Such alterations include methylation and (de)acetylation. Similar to DNA methylation, histone modifications can result in different epigenetic outcomes, depending on the genomic region of interest. In the case of histone methylation, the effects also depend on the number of methyl groups attached to histones, as this process is associated with both gene activation and repression (Golubeva et al., 2024; Jawaid et al., 2018). One of the most studied histone modifications is the acetylation of lysine (K) residues, which is catalysed by histone acetyltransferase (HAT), an enzyme that adds acetyl groups to histones, resulting in euchromatin and therefore in active gene expression. Conversely, in histone deacetylation an enzyme known as histone deacetylase (HDAC) removes the acetyl groups added by the HATs, causing a stronger affinity between DNA and the histones, thereby preventing gene transcription (Howie et al., 2019).
Numerous histone post-translational modifications appear to be affected by both acute and chronic stress, with important stress-induced cascades, such as corticosterone signalling, contributing to their regulation so as to modulate gene expression and, consequently, behavioural responses (Jawaid et al., 2018). This is consistent with the view of Howie et al. (2019), which states that histone regulation is involved in many brain processes, such as traumatic memory encoding and fear extinction, which are dysregulated in PTSD. More specifically, histone acetylation and phosphorylation (i.e., the addition of phosphate groups to histones, primarily associated with transcriptional activation (ScienceDirect, 2024)), similar to DNA methylation discussed above, are critical for gene regulation linked to fear memory. As this process is heavily involved in PTSD, its dysregulation through histone modifications can lead to the disorder’s symptoms. For instance, in rodents, the administration of HDAC inhibitors, which are therapeutic agents that prevent the removal of acetyl groups from histones (Zain, 2012), led to increased H3 acetylation in the amygdala and enhanced auditory fear memory. Similarly, HDAC inhibition in the hippocampus was found to modulate contextual fear (Cao-Lei et al., 2022). These findings indicate a close connection between histone modifications and fear memory regulation, also showing their link to PTSD.
2.1.4 NONCODING RNAS
Noncoding RNAs (ncRNAs) are DNA sequences, which are not translated into proteins and play a vital role in gene regulation and potentially in PTSD. They are involved in the regulation of other RNA molecules, such as messenger RNA (mRNA), transfer RNA (tRNA) and ribosomal RNA (rRNA) (Howie et al., 2019). Depending on the number of bases they consist of, they are divided into short ncRNAs (usually consisting of less than 200 nucleotides) and long ncRNAs (typically consisting of more than 200 nucleotides). Short ncRNAs (sncRNAs), specifically microRNAs (miRNAs), act by degrading mRNA or by translational repression. Long ncRNAs (lncRNAs) are associated with either gene activation or silencing, due to their interplay with chromatin, histones and RNA polymerase (Golubeva et al., 2024; Jawaid et al., 2018).
miRNAs, which are abundant in the brain, have been implicated in its susceptibility to trauma, as several studies suggest their potential involvement in the moderation of the effects of traumatic stress in PTSD patients (Jawaid et al., 2018). For instance, microRNA-124, which is the most highly expressed miRNA in the brain and has a central role in the modulation of signalling molecules underlying synaptic plasticity and memory (Sun et al., 2015), has been implicated in PTSD. Specifically, a study by Chen et al. (2022) showed that miR-124 levels in the hippocampus of rats exposed to single-prolonged stress (SPS) (i.e., an animal model for PTSD-like symptoms (Huang et al., 2023)) were downregulated, and that restoring miR-124 expression could alleviate the PTSD-like behaviours of SPS rats (Chen et al., 2022). In rodents, miRNAs also appear to have a role in regulating GR function, thereby influencing their behavioural response to stress. As for lncRNAs, they are also involved in regulation of gene expression in PTSD, although further research is required (Howie et al., 2019).
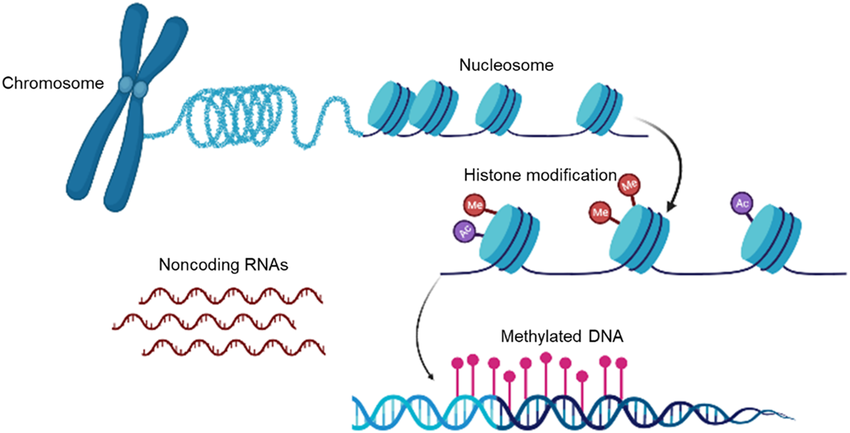
Figure 2: A simple overview of the three most studied epigenetic mechanisms relevant to PTSD: DNA methylation, histone modifications and noncoding RNAs. Taken from https://www.researchgate.net/figure/Mechanisms-of-epigenetics-modifications-Gene-expression-is-regulated-by-genetic-as-well_fig1_370803095.
To summarise, epigenetic mechanisms including DNA methylation, histone modifications and noncoding RNAs are closely linked to the alterations observed in PTSD. While significant progress has been made, particularly regarding DNA methylation and its impact on the HPA axis, further research is needed to fully understand their roles in the disorder.
2.2 EPIGENETIC INHERITANCE AND PTSD: EFFECTS OF PRECONCEPTION, PRENATAL AND POSTNATAL PARENTAL TRAUMA ON OFFSPRING PTSD RISK
Trauma has a particularly strong impact on the brain during critical developmental periods, when it is highly sensitive to environmental stimuli, owing to neurogenesis, the immaturity of the HPA axis and neuroendocrine interactions. These sensitive time windows concern prenatal, early postnatal and pubertal periods (Jawaid et al., 2018). However, studies have shown that parental traumatic exposure in the preconception period also significantly shapes the offspring’s risk of developing PTSD, with paternal and maternal trauma representing distinct risk factors (Yehuda & Lehrner, 2018). This supports the concept of epigenetic inheritance, as effects of parental traumatic experiences during the mentioned periods are believed to be passed down via the germline, influencing the stress response and PTSD risk in exposed individuals’ direct descendents or in subsequent generations not directly exposed to the events. The first case of transmission mentioned refers to intergenerational inheritance, on which we will primarily focus, and the second to transgenerational epigenetic inheritance, which concerns the F2 generation in males and the F3 generation in females (Chou et al., 2024).
The elevated prevalence of PTSD among offspring with parental PTSD, observed in the studies on Holocaust survivors and their descendents, gave rise to the possibility of biological risk factors shaping an individual’s susceptibility to the disorder (Yehuda & Lehrner, 2018). In this section, prompted by Yehuda and Lehrner (2018), we will discuss the inter- and potentially transgenerational effects of maternal and paternal trauma exposure on offspring risk of developing PTSD, focusing on prenatal maternal trauma, and preconception and postnatal parental trauma. The mechanisms that allow the epigenetic changes induced by these events to affect the parental germline, and in turn be passed down to future generations, will be addressed later in this paper.
2.2.1 PRENATAL MATERNAL TRAUMA
Prenatal maternal traumatic exposure can contribute to profound effects on offspring, increasing their risk of developing neuropsychiatric disorders, including PTSD (Klengel et al., 2015). The gestational period is believed to be critical for foetal programming, as the gestating embryo is highly sensitive to environmental stimuli to which the maternal lineage may be exposed (Klengel et al., 2015). This is attributable to the fact that the intrauterine environment constitutes a developmentally crucial context, through which maternal trauma can influence the programming of the HPA axis. This response system, although developed by 22 weeks of gestation, is still particularly vulnerable to environmental influence. Responsible for the regulation of maternal glucocorticoids, and therefore foetal protection, is the placenta. This happens through the expression of 11β-HSD2, an enzyme that converts cortisol into inactive cortisone (Holmes et al., 2006; Yehuda & Lehrner, 2018). Interestingly, prenatal stress in animal models was shown to reduce the activity of this enzyme, due to increased methylation of 11β-HSD2 in the placenta. This suggests that insufficient foetal protection against glucocorticoids could have significant consequences for glucocorticoid sensitive systems in the offspring, such as the HPA axis. These alterations have been heavily associated with PTSD (Yehuda & Lehrner, 2018).
Intrauterine traumatic exposure has also been found to contribute to offspring PTSD vulnerability through aberrant methylation in the NR3C1 promoter. This was observed in mothers exposed to the Tutsi genocide during pregnancy and their children, who both exhibited increased NR3C1 methylation (Chou et al., 2024). Similar cases concern offspring of mothers who were gestating at the time of 9/11 terrorist attacks and developed PTSD as a result. Their children presented with low cortisol levels, indicating that maternal traumatic exposure influenced the development of the offspring’s HPA axis (Klengel et al., 2015). Such examples demonstrate the profound impact that prenatal stress can have on foetoplacental interactions and thereby on offspring, especially regarding their susceptibility to psychiatric disorders.
2.2.2 PRECONCEPTION MATERNAL TRAUMA
In humans, parental preconception trauma has been associated with alterations in the HPA axis of their children (Duffy et al., 2021). Maternal preconception stress, especially in early childhood, but also throughout the lifespan, has been associated with effects on the oocytes and the uterine environment, which could potentially affect the offspring and their susceptibility to psychopathologies (Chou et al., 2024). Nonetheless, the field continues to be explored, as it is difficult to distinguish between the effects of maternal preconception stress in oocytes and the foetoplacental environment (Yehuda & Lehrner, 2018). As a result, this complicates the discernment of the route of transmission (Chou et al., 2024).
Despite these challenges, there have been studies that have investigated the correlation between maternal stress and mental health prior to conception, and its effects on offspring stress regulation. One example is the study conducted by Guardino et al. (2022) that found a positive link between preconception maternal mental health and diurnal cortisol slopes in early childhood. This was coupled with the significant observation that preconception maternal PTSD was associated with flatter diurnal cortisol slopes at the ages of 4 and 5, which were linked to long-term negative effects. Another study in this area, conducted by Swales et al. (2023), found that greater severity of preconception maternal PTSD symptoms predicted increased negative affectivity in early childhood, which is an indicator for later psychopathologies.
There have also been several relevant studies on Holocaust survivors and their offspring. For instance, a study conducted by Yehuda et al. (2008) showed that maternal PTSD was related to increased risk for PTSD in adult offspring of Holocaust survivors. Further research conducted by Yehuda et al. (2014) found that maternal PTSD moderated the impact of paternal PTSD, increased the offspring’s susceptibility to the disorder and was linked to the regulation of the NR3C1 gene in the descendants of Holocaust survivors.
2.2.3 PRECONCEPTION PATERNAL TRAUMA
A growing body of literature has focused on the transmission of paternal preconception trauma to offspring through sperm, as paternal exposure to preconception stress can impact the gametes at any stage of development during the individual’s lifetime. Additionally, paternal transmission also excludes foetoplacental interactions and maternal care (Chou et al., 2024; Yehuda & Lehrner, 2018). Mechanisms that have been implicated in this process and could be potential candidates for intergenerational and transgenerational transmission of paternal trauma are DNA methylation, histone modifications and ncRNAs, especially miRNAs that have a role in early spermatogenesis and adult sperm cells (Klengel et al., 2015). These will be addressed further below.
A representative case of the effects of preconception paternal stress on offspring is the exposure of male mice to chronic variable stress during puberty or adulthood. This exposure resulted in decreased responsiveness of the HPA axis and changes in the transcription of GR genes (Klengel et al., 2015; Rodgers et al., 2013). A further example is the study on adult male mice (F0 generation) that were exposed to an olfactory fear conditioning paradigm (Dias & Ressler, 2013). Both the F1 and F2 generations exhibited enhanced sensitivity to the same odour that the F0 mice were exposed to, indicating that the behavioural and neuroanatomical effects were transmitted in a transgenerational manner. The explanation provided involves the transmission of the observed sensitivity via ncRNAs and the maintenance of decreased methylation in the M71 odourant receptor (Klengel et al., 2015). Other findings, reported in the previously mentioned Holocaust survivor study conducted by Yehuda et al. (2014), include higher GR-1F promoter methylation (i.e., the promoter for the NR3C1 gene) in the offspring of PTSD-affected fathers. This modification possibly suggests alterations in the HPA axis feedback, as it leads to the reduction of GR expression, which impacts the offspring’s GR sensitivity and, consequently, their stress response.
2.2.4 POSTNATAL TRAUMA
The early postnatal period constitutes another critical time window for offspring development. Environmental perturbations in the postnatal environment can heavily impact mental health outcomes later in life (Klengel et al., 2015). Specifically, early-life traumatic stress can result in enduring transgenerational effects, causing pathologies such as psychosis and depression (Gapp et al., 2018). It is also worth noting that adverse childhood experiences (ACE) have been associated with psychiatric disorders, including PTSD (Chou et al., 2024). Although in humans it is difficult to examine the relation of postnatal trauma to heritable epigenetic changes while ruling out social transmission, rodent models provide compelling evidence for the epigenetic inheritance of postnatal trauma.
A recent study demonstrating the influence of postnatal environments on future generations is the above-mentioned investigation of the differences between rats of high and low maternal care, conducted by Weaver et al. (2004). However, another characteristic example is the MSUS model, which involves maternal separation combined with unpredictable stress in mice (Franklin et al., 2010; Gapp et al., 2014). This study showed that male mice (F1 generation) that experienced early postnatal separation and exhibited a depressive-like behaviour, produced female offspring with a similar depressive-like behaviour, which was also observed in the subsequent (F3) generation in males, along with altered DNA methylation patterns and upregulated miRNA levels. This study therefore exemplifies how early-life traumatic experiences can affect future generations through epigenetic transmission (Klengel et al., 2015).
To conclude, parental stress exposure can heavily influence the offspring and their susceptibility to psychopathologies, primarily by affecting their neurodevelopment and stress response, which is strongly linked to the risk of PTSD development.
2.3 TRAUMA-INDUCED EPIGENETIC CHANGES IN THE PARENTAL GERMLINE
Epigenetic inheritance, as already mentioned, refers by definition to the transmission of epigenetic changes via the germline (Jawaid et al., 2018). However, in order to refer to epigenetic germ cell transmission (Duffy et al., 2021), the means by which the trauma-induced epigenetic alterations affect the germ cells, resulting in offspring phenotype, must first be discussed. Although inheritance across generations can occur through both matrilineal and patrilineal lineages (Jawaid et al., 2018), the existing literature almost primarily focuses on the trauma-related epigenetic modifications affecting sperm, as it is difficult to parse different maternal contributors to offspring outcome (Yehuda & Lehrner, 2018). Therefore, in this section of the review, we will shortly refer to the first study examining the effect of preconception stress on oocytes, conducted by Zaidan et al. (2013). We will also attempt to summarise existing literature on epigenetic changes induced by mental disorders in sperm and address the suggested means that potentially explain this process.
2.3.1 EPIGENETIC CHANGES IN OOCYTES
As mentioned above, in 2013 Zaidan et al. conducted the first study assessing the effect of preconception stress on oocytes. During this study, adult female rats were exposed to chronic unpredictable stress. Greater oocyte expression of corticotropin-releasing factor 1 (CRF1) mRNA was observed in the oocytes of the stress-exposed rats compared to control rats. Importantly, adult offspring of the stress-exposed female rats also exhibited group differences in CRF1 expression in the amygdala and frontal cortex. However, these differences were additionally dependent on the stress exposure of the progeny, and this study therefore does not provide mechanistic evidence for the influence of maternal preconception stress on oocytes as it did also not control for the intrauterine environment and maternal behaviour (Duffy et al., 2021). Nevertheless, a follow-up study conducted by Zaidan et al. (2021) expanded on these findings, identifying altered miRNA expression patterns in the oocytes of female rats exposed to pre-reproductive stress. These changes were also found to have affected the neonate brain of both the F1 and F2 generations, proposing microRNA modifications in stress-exposed oocytes as a possible mechanism for epigenetic inheritance.
2.3.2 EPIGENETIC CHANGES IN SPERM
Epigenetic signatures of male gametes involve DNA methylation, histone post-translational modifications (HPTMs) and ncRNAs. It should be noted that exposure to the same environmental stressor can potentially lead to multiple types of epigenetic modifications in sperm (Tan et al., 2023).
DNA METHYLATION
In sperm, DNA can be differentially methylated by environmental factors at different loci (Jawaid et al., 2018) and this has been implicated in paternal transmission (Tan et al., 2023). Evidence from rodent models indicates that traumatic experiences can alter DNA methylation patterns in male mouse sperm, and unstressed offspring seem to exhibit similar modifications in certain tissues. For instance, in the MSUS model, modifications in DNA methylation patterns of exposed mice were found, and the same differentially methylated regions were observed in the brains of their direct descendants (Franklin et al., 2010; Tan et al., 2023).
HISTONE POST-TRANSLATIONAL MODIFICATIONS
Although the contribution of histones to transgenerational epigenetic inheritance appears to be limited, environmental stressors can also modulate HPTMs. This observation has been made, for instance, in C. Elegans (Jawaid et al., 2018). However, it should be noted that no studies have provided compelling evidence for the influence of paternal traumatic experiences on histone PTMs in sperm (Tan et al., 2023).
NONCODING RNAS
The role of ncRNAs in sperm has recently been extensively studied and is supported by causal evidence that suggests their involvement in epigenetic inheritance, as sperm small ncRNAs appear to be a substrate for transgenerational transmission (Duffy et al., 2021) and participate significantly in foetal programming (Rodgers & Bale, 2015). Recent research has shown the variety of sncRNAs present in sperm, including miRNAs, which are susceptible to chronic stress (Tan et al., 2023). In addition to rodent studies (e.g., Rodgers et al., 2013), human studies have also indicated that traumatic events are associated with ncRNA modifications in sperm. For example, men exposed to early adverse childhood experiences (ACEs) exhibited reductions in sperm miR-449a and miR-34c levels (Dickinson et al., 2018). As these types of RNA are not present in oocytes, but are delivered to them via sperm during fertilisation, this suggests the potential influence of these miRNA on early offspring development and their implication in epigenetic inheritance (Duffy et al., 2021).
2.3.3 AFFECTING THE GERMLINE
Although glucocorticoids appear to be associated with epigenetic alterations in sperm and with offspring phenotypes resembling those observed in paternal stress models (e.g., Franklin et al., 2010), the precise mechanism by which traumatic stress affects the germline epigenome remains largely unknown (Jawaid et al., 2018). However, there are plausible mechanisms that may account for this phenomenon. These are related to circulating, lipid soluble transposable factors, such as hormones, cytokines or circulating RNAs, which can potentially access germ cells despite the protection of the blood-testis barrier, and consequently mediate the effects of trauma (Jawaid et al., 2018).
There is evidence for small RNAs acting as information carriers throughout the body, communicating stress signals to different tissues and the germ cells, specifically sperm (Klengel et al., 2015). Circulating RNAs are mainly transported in extracellular vesicles (EVs) within the caput epididymal lumen, called exosomes (Jawaid et al., 2018). Exosomes encode environmental challenges through changes in their sncRNA content, which could also be altered by other factors, such as distal somatic cells. As sperm transit through the epididymis during maturation, they acquire epigenetically altered ncRNAs from exosomes (Duffy et al., 2021; Jawaid et al., 2018), which could then be transferred to the oocyte at fertilisation, consequently affecting gene expression in the early embryo (Yehuda & Lehrner, 2018). Thus, small ncRNAs could function as long-term signals to the gametes (Klengel et al., 2015), affecting somatic epididymal epithelial cells, which retain stress-induced epigenetic changes and are able to encode and signal stress in the absence of the initial stimulus (Duffy et al., 2021). Supporting this hypothesis is a recent study conducted in mice by Chan et al. (2020). Using intracytoplasmic sperm injection, the study showed that sperm exposed to EVs derived from stress-exposed epididymal epithelial cells, gave rise to offspring with altered neurodevelopment and modified adult stress responses (Chan et al., 2020; Duffy et al., 2021). EVs have also been shown to affect the gene regulation of female reproductive tract cells, thereby potentially affecting offspring health (Tan et al., 2023).
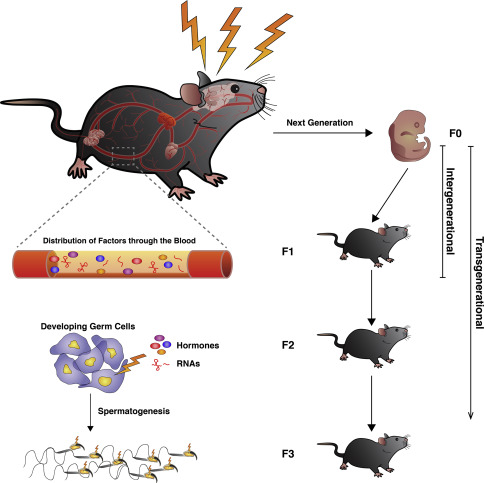
Figure 3: Potential mechanisms for the onset and maintenance of the transgenerational effects of trauma. Image and description taken from https://www.sciencedirect.com/science/article/abs/pii/S187711731830053X.
In summary, the mechanisms through which trauma affects the germ cells remain under investigation, with several aspects of this process still not fully understood. Advancing our knowledge in this area is crucial, as it may help clarify how PTSD impacts the germline and contributes to the epigenetic transmission of its risk factors.
2.4 POTENTIAL MECHANISMS FOR EPIGENETIC INHERITANCE AND PERSISTENCE OF PTSD-INDUCED EPIGENETIC CHANGES
Although there are several studies on the intergenerational effects of PTSD in humans, few are those that focus on the transgenerational transmission of this disorder, as human studies on this topic suffer from several limitations, which will be discussed in Section 4 of this review. In order to provide compelling evidence for the epigenetic inheritance of trauma across generations, the transmission of trauma-induced epigenetic changes via the germline must be established (Chou et al., 2024). The inheritance of epigenetic marks is only feasible if they survive epigenetic reprogramming, which refers to the genome-wide erasure and reestablishment of chemical tags (Clark & Rager, 2020). This process first occurs during gametogenesis, where demethylation establishes the totipotency of the zygote, enabling cell differentiation. It reoccurs during embryogenesis, when primordial germ cells (PGCs) undergo a second wave of demethylation. Restoration of methylation markers happens through de novo methylation, first after the implantation of the developing blastocyst, and again after sex determination (Lacal & Ventura, 2018). However, reprogramming does not occur universally, as certain epigenetic marks are not erased, which results in their transmission to offspring and potentially to future generations (Migicovsky & Kovalchuk, 2011). For this reason, genomic imprinting (i.e., the regulatory process in which one allele of a gene is expressed and the other is epigenetically repressed due to methylation (National Human Genome Institute, 2025)), is currently under investigation, as imprinted genes, along with some subsets of retrotransposons, such as intracisternal A particles (IAPs) in mice, survive epigenetic reprogramming, which may provide valuable insights into the inheritance of other epigenetic marks (Migivovsky & Kovalchuk, 2011).
In this section, we will discuss current evidence concerning the survival of specific epigenetic marks during epigenetic reprogramming, their role in the inheritance of trauma and potentially of PTSD risk, and how they may regulate brain development and exert long-term effects on offspring stress reactivity after impacting the parental germline (Rodgers & Bale, 2015). Again, we will primarily focus on sperm, as evidence for transmission through the male germline is characteristically dominant.
2.4.1 DNA METHYLATION, HISTONE MODIFICATIONS AND NONCODING RNAS AS MEANS OF EPIGENETIC INHERITANCE
Methylation in sperm DNA has been characterised as a potential substrate for transgenerational transmission, because, as mentioned previously, alterations in methylation patterns in sperm, following early-life stress, have been linked to offspring phenotypes (Rodgers & Bale, 2015). These modifications are heritable through maintenance DNA methylation (Klengel et al., 2015). Although thought to be completely erased during reprogramming, methylation marks at certain loci, including imprinted genes and repetitive elements were found to be able to evade epigenetic erasure (Rodgers & Bale, 2015). This makes it conceivable that other loci may as well survive reprogramming. It is also suggested that repetitive elements, which are present in the genome, potentially serve as transmitters of epigenetic information, thereby influencing the expression of other genes (Klengel et al., 2015). As for imprinted genes, sperm differentially methylated regions (DMRs) may survive reprogramming with the help of two potential mechanisms, one of them suggesting locus-specific retention and the other referring to their inheritance by reconstruction after erasure in contrast to non erasure (Tan et al., 2023). This also refers to the idea of replicative and reconstructive transmission (Fitz-James & Cavalli, 2022).
Another potential platform for epigenetic inheritance is histone modifications. Although histones also undergo large-scale changes during gametogenesis and fertilisation, as with DNA methylation, it was found that several histone regions retain their identity, especially those of silenced chromatin (Fitz-James & Cavalli, 2022). One of the objections against histone-mediated inheritance is the replacement of the majority of histone proteins by protamines in sperm. However, it was established that this replacement is incomplete, as approximately 4% to 15% of histones were found to remain in human sperm, and 1% in mice sperm (Migicovsky & Kovalchuk, 2011). This suggests the potential transmission of epigenetic information through the sperm histone code (Klengel et al., 2015). Specifically, remaining histones bear modifications that are both shaped by the paternal chromatin environment and serve as precursors to gene expression patterns in the early embryo (Fitz-James & Cavalli, 2022). This is attributable to the fact that they primarily occur at genomic regions critical to embryogenesis (Rodgers & Bale, 2015), resulting in the paternal genome not only providing DNA sequence, but also molecular regulatory factors (Migicovsky & Kovalchuk, 2011). This could also suggest the influence of paternal stress on post-translational modifications of retained histones, which, nevertheless, requires further research (Rodgers & Bale, 2015).
Sperm RNA populations have also been implicated in epigenetic transmission. Several studies (e.g., Gapp et al., 2018) have shown that substantial quantities of ncRNAs are transmitted to the next generation via the germline, especially through sperm (Fitz-James & Cavalli, 2022). In mammalian sperm, changes in RNA populations have been implicated in the programming of the offspring’s HPA axis and stress reactivity. Moreover, due to their integral role in embryogenesis, small ncRNAs are considered key drivers of transgenerational reprogramming (Rodgers & Bale, 2015). Supporting this, purified RNA from the sperm of stressed rodent sires was injected into zygotes and was found to reproduce core features of the offspring phenotype (Gapp et al., 2014). Additionally, miRNAs are also important to embryogenesis, as they selectively degrade maternal mRNA (Rodgers & Bale, 2015) and have been shown to modulate de novo methylation in mouse embryonic stem cells (Sinkkonen et al., 2008). This indicates that even minor environmentally-induced changes in miRNA can significantly impact foetal development (Rodgers & Bale, 2015).
As discussed earlier, RNA-loaded exosomes can transmit information to germ cells based on the environment that the organism has encountered, which is then delivered to the oocytes during fertilisation. This may also contribute to paternal epigenetic transmission (Duffy et al., 2021). Furthermore, piwi-associated interfering RNAs (piRNAs) are also viewed as a crucial form of epigenetic memory. In fact, a study on a Chinese population by Zhu et al. (2016) showed that they can be passed on through epigenetic inheritance in humans (Migivovsky & Kovalchuk, 2011). This type of ncRNAs is primarily expressed in spermatids and has been linked to transgenerational transcriptional silencing in C. Elegans, but its role in mammals remains under investigation (Ashe et al., 2012).
As for the epigenetic transmission of stress, it is thought that sncRNAs modulate offspring development by triggering a sequence of molecular processes that transmit the stress signal. For instance, a specific miRNA, miR-34c, is implicated in the epigenetic transmission of stress through sperm (Dickson et al., 2018; Howie et al., 2019). Moreover, tsRNAs, which are regulatory ncRNAs derived from tRNAs (Zhang et al., 2023), have also been associated with stress-induced epigenetic inheritance, as demonstrated by the study conducted by Zheng et al. (2021). In this study, long-term paternal stress in mice was found to alter sperm tsRNAs, along with other small RNAs, including miRNAs and rsRNAs (i.e., ribosomal-derived small RNAs (Kalakoti et al., 2023)), which were associated with the epigenetic inheritance of stress-related traits across generations, through the regulation of methylation patterns. This further suggests that even subtle modifications in sperm sncRNAs could impact embryonic development and offspring phenotype, which highlights their involvement in stress signal transmission (Duffy et al., 2021). Finally, RNA is widely regarded as a heritable molecule capable of regulating other epigenetic processes, such as chromatin modification, reinforcing its significant role in epigenetic inheritance (Švorcová, 2023).
2.4.2 EPIGENETIC MARKS AND OFFSPRING NEURODEVELOPMENT
Concerning PTSD and the epigenetic inheritance of other psychopathologies, it remains largely unclear how epigenetic modifications in the germline affect specific cell types within a multicellular organism (Klengel et al., 2015). This includes uncertainty about how the effects of traumatic stress are transmitted from the germline to the offspring’s brain, influencing neurodevelopment and particularly the programming of the HPA axis and behavioural stress response. Epigenetic marks in germ cells may be sustained throughout development and into the adult brain, where they affect behaviour and physiology (Jawaid et al., 2018; Rodgers & Bale, 2015). For instance, in the MSUS model, similar epigenetic alterations were observed in the sperm of trauma-exposed mice and the brain of their female offspring, along with miRNA persistence (Gapp et al., 2014). Importantly, the precise activation of sperm epigenetic machinery observed at loci involved in the regulation of the HPA axis indicates a specific targeting mechanism, which controls brain development and is not only broadly involved in transcriptional regulation (Rodgers & Bale, 2015). Furthermore, as gametes form the zygote, an epigenetic change that persists from sperm into neurons may propagate broadly, affecting multiple somatic tissues. However, differential maintenance of epigenetic marks resulting in brain region-specific outcomes is also a plausible hypothesis. Finally, regarding the persistence of these epigenetic alterations in brain and sperm, it is likely that trauma-induced changes are converted into other epigenetic or even genetic changes, due to genomic instability (Jawaid et al., 2018).
To conclude, DNA methylation, histone modifications and noncoding RNAs are the most extensively studied mechanisms of epigenetic transmission in mammals. Their capacity to persist through epigenetic reprogramming while maintaining proper development (Migicovsky & Kovalchuk, 2011) enables environmental experiences, such as trauma, to impact future generations. Moreover, the close relationship between the germline epigenome and brain development suggests that epigenetic marks can influence offspring neurodevelopment, including HPA axis reactivity. This may help explain the epigenetic transmission of PTSD risk across generations.
3. CASE STUDIES AND HUMAN EVIDENCE
It is a proven fact that certain historical events and PTSD have a connection, both mentally and genetically. This section aims to break this connection down by showing real life incidents and highlighting their effects on their victims and the offsprings of their victims. The Holocaust and 9/11 attacks are going to be the prime examples of this phenomenon. The Holocaust is the systematic state-sponsored killing of six millions of others by Nazi Germany and its collaborators during World War ll (Berenbaum, 2025). On the other hand, 9/11 is a series of airline hijackings and suicide attacks committed in 2001 by militans associated with al-Qaeda against targets in the United States (Bergen, 2025).
With few exceptions, the literature on adult offspring of Holocaust survivors is divided into two camps: descriptions of the adverse effects of the Holocaust on the second generation and failures to find such effects. Initial reports described an unusually high incidence of depression, anxiety, conduct disorder, personality problems, inadequate maturity, excessive dependence on substances and poor coping skills in the children of Holocaust survivors. In addition, offspring of Holocaust survivors were described as having a general fragility and vulnerability to stress. Research has also shown that symptoms of post-traumatic stress disorder (PTSD) are the most common health effect of the 9/11 attacks. Up to 20% of adults directly exposed to the disaster or injured in the attack had PTSD symptoms five to six years after the attack; this is four times the rate in the general population.
3.1 9/11 AND ITS UNAVOIDABLE EFFECTS
Researchers at Columbia University led the first effort to track the mental health of people who witnessed the 9/11 attacks, surveying them in the weeks after the disaster and following them for three years. Dr Sandro Galea explained that his team initially conducted a study focused on Manhattan and later secured funding to expand their research to include New York, southern Connecticut and eastern New Jersey. Their early findings showed that the rates of depression and post-traumatic stress disorder in the general population had roughly doubled compared to the usual levels.
Studies of those enrolled in the World Trade Center Health Registry also show that most people with symptoms are often struggling with multiple physical and/or mental health conditions. Dr Rothbaum noted that many individuals try to manage their symptoms on their own, often using marijuana to help them sleep. However, this approach can eventually lead to additional problems. Around 400,000 people directly affected by the attack on the twin towers are eligible to be enrolled in the World Trade Center Health Registry. Enrollees can be referred to the World Trade Center Health Program, which provides free monitoring and health care to those directly affected by the disaster (Chatterjee, 2021).
3.2 THE HOLOCAUST AND ITS UNAVOIDABLE EFFECTS
In 1948, psychiatrist Paul Friedman described what he termed “Buchenwald Syndrome” (named for the concentration camp Buchenwald), a collection of psychological symptoms exhibited by camp survivors. Danish researcher Per Helweg-Larsen, describing a similar set of symptoms, coined the term “survivor syndrome” in 1952, and in 1954 Knud Hermann and Paul Thygesen coined the term “Konzentrationslagersyndrom” (KZ-Syndrom, or concentration camp syndrome). All refer to the mental health disorders that many Holocaust survivors developed as a result of the physical and psychological trauma they suffered. These included sleep disorders, concentration disorders, irritability, anxiety, night terrors, phobias and flashbacks. This is further supported by transgenerational transmission, which was developed through a Canadian clinic in 1966, where it was observed that the patients whose parents or grandparents were survivors of the Holocaust were more likely to have a mental disorder. Children of Holocaust survivors appear to be plagued with feelings of guilt or responsibility for their parents, and there is some evidence that this sense of guilt is also heritable: this is being explored through transgenerational transmission epigenetics that found that stress genes are passed down generationally. Moreover, Moshe Szyf, in an article titled “The trauma may be inherited”, noted that children and grandchildren of survivors “have higher rates of post-traumatic stress after enduring car accidents, possibly due to modifications in their stress hormone system inherited from their survivor parents”. Children were also known to have vivid nightmares of the Holocaust despite never experiencing it, showing the connection to later generations developing PTSD symptoms.
3.3 CONCLUSION
The impact of historical events on mental health outcomes is not limited to war-related experiences; it can also extend to other collective traumatic events, such as famines and natural disasters. Therefore, future research should expand its focus to other regions and populations worldwide that have been exposed to significant historical traumas. Overall, studies show the survivors of these events are likely to pass on the impacts of extreme trauma to their children and grandchildren through their genetics: emotionally, through PTSD symptoms and mental disorders, and physically, through stress modifiers in their genes. Hopefully more research will be done, so that we can better understand how these events affect our understanding of trauma and the generational implications of it.
4. LIMITATIONS AND SCIENTIFIC CONTROVERSY
Epigenetic inheritance in a person who has PTSD involves changes in DNA methylation that are found in stress-related genes such as FKBP5 and NR3C1. The children of trauma survivors have shown similar changes in these genes, showing that PTSD can be biologically passed down. Most epigenetic marks are reprogrammed during reproduction, but some marks may not, causing the genes to be passed down to their children (Heard & Martienssen, 2014). However, proving epigenetic inheritance in humans is very difficult. Changes in methylation linked to trauma may appear in both a parent and their child, however that does not necessarily mean they were inherited biologically. These changes may have been caused by exposure, such as growing up in a stressful home or with a traumatised parent (Hurley, 2015), making inheritance hard to prove. A child may develop PTSD symptoms from the behaviour of their parents, which may include overprotectiveness, anxiety or emotional distance, yet this can vary from person to person. This is called behavioural transmission and it is difficult to distinguish from epigenetic inheritance (Wikipedia, 2024). Studies have also shown that stress from childhood abuse, neglect, violence, racism or war can change gene expression without a family history of trauma. This means new epigenetic changes can occur from personal experiences rather than inheritance. Additionally, not everyone responds to trauma the same way due to factors such as personality, support systems and resilience, making it even more complex to track genetic changes.
While epigenetic markers like DNA methylation and histone modification can affect the expression of genes linked to stress responses (like NR3C1 and FKBP5), it is hard to prove that these changes are an epigenetic change. Children of people with PTSD often grow up in high stress environments, exposed to their parents emotional responses, coping mechanisms and trauma-related behaviours. This makes it hard to determine whether it is a result of inherited epigenetic changes or learnt behaviour through upbringing (Heard, 2014). For example, studies found that Holocaust survivors and their children shared similar epigenetic modifications in stress-related genes. However, those same children also grew up hearing traumatic stories or observing trauma-related behaviour from their parents. This makes it hard to distinguish whether it’s behavioural transmission, inheritance or the environment they grew up in. Studies on the children of people exposed to war or severe violence have shown altered cortisol levels and gene expression patterns but proving the cause is still very difficult (Hurley, 2015). Even animal studies show that behavioural interaction can lead to effects similar to inherited ones: for example, mice that are raised by stressed mothers develop anxiety even if they aren’t genetically related, simply because of behavioural transmission (Heard & Martienssen, 2014).
One of the biggest challenges in studying epigenetic inheritance in humans is the lack of evidence due to ethical and practical restrictions. Scientists cannot ethically intentionally expose humans to trauma, meaning the majority of research studies rely on historical events, where conditions cannot be controlled (Dias & Ressler, 2014), or animal models, which do not perfectly replicate human gene expression (Skvortsova et al., 2018). Additionally, researchers cannot easily study sperm and egg cells in living humans, especially in large numbers. These are the cells that pass information to the next generation, adding a further challenge (Meaney, 2010).
5. IMPLICATIONS, APPLICATIONS AND FUTURE RESEARCH
Animal models have successfully demonstrated the epigenetic mechanisms of transgenerational inheritance. However, it would be extrapolation to use these studies to conclude that these mechanisms of transgenerational inheritance of trauma also occur in humans; further evidence is necessary. Future research should be longitudinal and multi-generational studies to account for inheritance only being considered transgenerational if it is observed in the absence of the stimulus (F2 generation in males and F3 generation in females). Future research should also take into account genetic and environmental understanding to ensure the research is truly assessing transfer of epigenetic marks. (Yehuda & Lehrner, 2018) This research is financially and temporally expensive, so for now the animal models provide promising evidence for the potential of human transgenerational inheritance of the effects of trauma through epigenetic marks.
Mice models have also shown the potential for the effects of trauma in parents and offspring to lead to resilience, active coping and adapting to a challenging environment (Lyons & Parker, 2007), as well as the psychopathology effects observed as a result of trauma. This has implications for the potential for reversibility of these impacts. The permanence of these acquired phenotypes is lacking once environmental conditions have changed, as behaviour and the epigenome are sensitive to the environment and can be modified across life. These concepts have implications in the public health industry for the design of diagnostic and therapeutic approaches for trauma-related psychopathologies (Gapp et al, 2016). Environmental enrichment (EE) can be used as a positive intervention to improve cognitive function and reduce anxiety-related behaviours through sensory and motor stimulation (Sampedro-Piquero & Begega, 2017). These EE therapies should be incorporated into trauma-informed care. They should also recognise the widespread, intergenerational impact of trauma and the potential recovery pathways. They should place an emphasis on physical and emotional safety and the prevention of re-traumatisation. Public health care systems should enforce guidelines for PTSD patients and their families to be recommended to experience trauma-informed, intergenerational support with these priorities in mind. Support and therapy should be offered across multiple generations, as epigenetics research has demonstrated the impact of trauma on children and grandchildren (Yehuda & Lehrner, 2018). Increasing the frequency of the prescription of EE therapy and trauma-informed care will decrease the over-reliance on pharmacological intervention and medication (Sampedro-Piquero & Begega, 2017). The findings of this paper also support the potential for introduction of early-intervention treatments for families that are at risk of the effects of trauma being inherited by subsequent generations.
6. CONCLUSION
Epigenetic inheritance of trauma is an emerging yet powerful field of research. The prevalence of the effects on offspring of individuals who experience traumatic events presents large implications for the treatment of PTSD patients and the potential to introduce early intervention for them and their families. Trauma can induce changes in gene expression without altering the underlying DNA sequence in the form of epigenetic marks. There is accumulating evidence in this field for the inheritance of trauma-related traits and the mechanisms that underpin the transmission of epigenetic marks such as DNA methylation, histone modifications and non-coding RNAs. Although they offer plausible pathways for intergenerational transmission, there are still significant methodological challenges that limit the generalisability of the concepts. Mice and rodent models have provided evidence for the mechanisms linked to PTSD and case studies such as the Holocaust, the 9/11 attacks and genocides throughout history have the potential to provide human evidence. However, epigenetic inheritance is very difficult to prove. There is difficulty in separating the influence of genetics, environmental factors and learnt behaviour as a result of growing up in high-stress environments and being exposed to parents’ emotional and behavioural consequences as a result of their trauma. Therefore it is difficult to identify if the consequences observed are truly a result of epigenetic inheritance. Ethical restrictions also limit the use of these case studies as evidence.
In the future, research should take on this understanding of the biological mechanisms and, through long-term studies, work to provide evidence for these mechanisms occurring in humans. Research into environmental enrichment therapy should act as a pointer for future directions of public health care, as it can act as early intervention for offspring affected by PTSD in their parents, in order to reduce the impact of the effects that could be transgenerationally inherited as a result of epigenetic marks.
Bibliography
Addissouky, T.A., El Tantawy El Sayed, I. & Wang, Y. (2025). Epigenetic factors in posttraumatic stress disorder resilience and susceptibility. Egyptian Journal of Medical Human Genetics, 26(1). [Available online: https://jmhg.springeropen.com/articles/10.1186/s43042-025-00684-w].
Antal, A., Chaieb, L., Moliadze, V., Monte-Silva, K., Poreisz, C., Thirugnanasambandam, N., Nitsche, M.A., Shoukier, M., Ludwig, H. & Paulus W. (2010). Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimulation, 3(4), pp. 230–237. [Available online: https://www.sciencedirect.com/science/article/abs/pii/S1935861X09001120#:~:text=The%20brain%2Dderived%20neurotrophic%20factor%20(BDNF)%20gene%20is,impaired%20in%20individuals%20expressing%20the%20Val66Met%20polymorphism].
Ashe, A., Sapetschnig, A., Weick, E.M., Mitchell, J., Bagijn, M.P., Cording, A.C., Doebley, A.L., Goldstein, L.D., Lehrbach, N.J., Le Pen, J., Pintacuda, G., Sakaguchi, A., Sarkies, P., Ahmed, S. & Miska E.A. (2012). piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell, 150(1), pp. 88–99. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3464430/].
Aykac, A. & Kalkan, R. (2021). Epigenetic approach to PTSD: In the aspects of Rat Models. Global Medical Genetics, 9(1), pp. 007–013. [Available online: https://www.thieme-connect.de/products/ejournals/html/10.1055/s-0041-1736633#JR2100041-1].
Bhattacharya, S., Fontaine, A., MacCallum, P.E., Drover, J. & Blundell, J. (2019). Stress Across Generations: DNA Methylation as a Potential Mechanism Underlying Intergenerational Effects of Stress in Both Post-traumatic Stress Disorder and Pre-clinical Predator Stress Rodent Models. Frontiers in Behavioral Neuroscience, 13, 113. [Available online: https://www.frontiersin.org/journals/behavioral-neuroscience/articles/10.3389/fnbeh.2019.00113/full].
Bierer, L.M., Bader, H.N., Daskalakis, N.P., Lehrner, A., Provençal, N., Wiechmann, T., Klengel, T., Makotkine, I., Binder, E.B., & Yehuda, R. (2020). Intergenerational effects of maternal holocaust exposure on FKBP55 methylation. American Journal of Psychiatry, 177(8), pp. 744–753. [Available online: https://psychiatryonline.org/doi/10.1176/appi.ajp.2019.19060618?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed].
Bowers, M.E. & Yehuda, R. (2015). Intergenerational transmission of stress in humans. Neuropsychopharmacology, 41(1), pp. 232–244. [Available online: https://www.nature.com/articles/npp2015247].
Cao-Lei, L., Saumier, D., Fortin, J. & Brunet, A. (2022). A narrative review of the epigenetics of post-traumatic stress disorder and post-traumatic stress disorder treatment. Frontiers in Psychiatry, 13. [Available online: https://www.frontiersin.org/journals/psychiatry/articles/10.3389/fpsyt.2022.857087/full].
Chan, J.C., Morgan, C.P., Adrian Leu, N., Shetty, A., Cisse, Y.M., Nugent, B. M., Morrison, K.E., Jašarević, E., Huang, W., Kanyuch, N., Rodgers, A.B., Bhanu, N.V., Berger, D.S., Garcia, B.A., Ament, S., Kane, M., Neill Epperson, C., & Bale, T.L. (2020). Reproductive tract extracellular vesicles are sufficient to transmit intergenerational stress and program neurodevelopment. Nature Communications, 11(1). [Available online: https://www.nature.com/articles/s41467-020-15305-w#Abs1].
Chen, Y., An, Q., Yang, ST., Chen, YL., Tong, L. & Ji, LL. (2022). MicroRNA-124 attenuates PTSD-like behaviors and reduces the level of inflammatory cytokines by downregulating the expression of TRAF6 in the hippocampus of rats following single-prolonged stress. Experimental Neurology, 356, p. 114154. [Available online: https://www.sciencedirect.com/science/article/abs/pii/S0014488622001790?via%3Dihub].
Chou, PC., Huang, YC. & Yu, S. (2024). Mechanisms of Epigenetic Inheritance in Post-Traumatic Stress Disorder. Life, 14(1). [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10817356/#sec3-life-14-00098].
Clark, J. & Rager, J.E. (2020). Epigenetics: An overview of CPG methylation, chromatin remodeling, and regulatory/noncoding RNAs. Environmental Epigenetics in Toxicology and Public Health, 3–32. [Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128199688000019].
Day, J.J. & Sweatt, J.D. (2010). DNA methylation and memory formation. Nature Neuroscience, 13(11), pp. 1319–1323. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3130618/].
Dias, B.G. & Ressler, K.J. (2013). Parental olfactory experience influences behavior and neural structure in subsequent generations. Nature Neuroscience, 17(1), pp. 89–96. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3923835/].
Dickson, D.A., Paulus, J.K., Mensah, V., Lem, J., Saavedra-Rodriguez, L., Gentry, A., Pagidas, K. & Feig, L.A. (2018). Reduced levels of miRNAs 449 and 34 in sperm of mice and men exposed to early-life stress. Translational Psychiatry, 8(1). [Available online: https://www.nature.com/articles/s41398-018-0146-2].
Du, J., Diao, H., Zhou, X., Zhang, C., Chen, Y., Gao, Y. & Wang, Y. (2022). Post-traumatic stress disorder: A psychiatric disorder requiring urgent attention. Medical Review, 2(3), pp. 219–243. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10388753/].
Duffy, K.A., Bale, T.L. & Epperson, C.N. (2021). Germ Cell Drivers: Transmission of Preconception Stress Across Generations. Frontiers in Human Neuroscience, 15. [Available online: https://www.frontiersin.org/journals/human-neuroscience/articles/10.3389/fnhum.2021.642762/full].
FHEHealth (2023). Are mental disorders increasing over time?. [Available online: https://fherehab.com/mental-health-disorders-increasing] (Accessed: 17th July 2025).
Fitz-James, M.H. & Cavalli, G. (2022). Molecular mechanisms of transgenerational epigenetic inheritance. Nature Reviews Genetics, 23(6), pp. 325–341. [Available online: https://www.nature.com/articles/s41576-021-00438-5].
Franklin, T.B., Russig, H., Weiss, I.C., Gräff, J., Linder, N., Michalon, A., Vizi, S. & Mansuy, I.M. (2010). Epigenetic transmission of the impact of early stress across generations. Biological Psychiatry, 68(5), pp. 408–415. [Available online: https://www.biologicalpsychiatryjournal.com/article/S0006-3223(10)00576-7/fulltext].
Gapp, K., Jawaid, A., Sarkies, P., Bohacek, J., Pelczar, P., Prados, J., Farinelli, L., Miska, E. & Mansuy, I.M. (2014). Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nature Neuroscience, 17(5), pp. 667–669. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4333222/].
Gapp, K., Bohacek, J., Grossmann, J., Brunner, A.M., Manuella, F., Nanni, P. & Mansuy, I.M. (2016). Potential of environmental enrichment to prevent transgenerational effects of paternal trauma. Neuropsychopharmacology, 41(11), pp. 2749–2758. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC5026744/].
Gapp, K., van Steenwyk, G., Germain, P.L., Matsushima, W., Rudolph, K.L.M., Manuella, F., Roszkowski, M., Vernaz, G., Ghosh, T., Pelczar, P., Mansuy, I.M. & Miska, E.A. (2018). Alterations in sperm long RNA contribute to the epigenetic inheritance of the effects of postnatal trauma. Molecular Psychiatry, 25(9), pp. 2162-2174. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7473836/].
Golubeva, E., Zeltser, A., Zorkina, Y., Ochneva, A., Tsurina, A., Andreyuk, D., Kostyuk, G. & Morozova, A. (2024). Epigenetic Alterations in Post-Traumatic Stress Disorder: Comprehensive Review of Molecular Markers. Complex Psychiatry, 10(1-4), pp. 71-107. [Available online: https://karger.com/cxp/article/10/1-4/71/916080/Epigenetic-Alterations-in-Post-Traumatic-Stress].
Guardino, C.M., Rahal, D., Rinne, G.R., Mahrer, N.E., Davis, E.P., Adam, E. K., Shalowitz, Madeleine. U., Ramey, S.L. & Schetter, C.D. (2022). Maternal stress and mental health before pregnancy and offspring diurnal cortisol in early childhood. Developmental Psychobiology, 64(7). [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10111814/].
Heard, E. & Martienssen, R. (2014). Transgenerational Epigenetic Inheritance: Myths and Mechanisms. Cell, 157(1), pp. 95–109. [Available online: https://www.cell.com/fulltext/S0092-8674(14)00298-4].
Hefner, K., Whittle, N., Juhasz, J., Norcross, M., Karlsson, R.M., Saksida, L.M., Bussey, T.J., Singewald, N. & Holmes, A. (2008). Impaired Fear Extinction Learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. The Journal of Neuroscience, 28(32), pp. 8074–8085. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC2547848/].
Holmes, M.C., Abrahamsen, C.T., French, K.L., Paterson, J.M., Mullins, J.J., & Seckl, J.R. (2006). The mother or the fetus? 11β-hydroxysteroid dehydrogenase type 2 null mice provide evidence for direct fetal programming of behavior by endogenous glucocorticoids. The Journal of Neuroscience, 26(14), 3840–3844. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6445356/].
Howie, H., Rijal, C.M. & Ressler, K.J. (2019). A review of epigenetic contributions to post-traumatic stress disorder. Dialogues in Clinical Neuroscience, 21(4), pp. 417-428. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6952751/].
Huang, G., Iqbal, J., Shen, D., Xue, Y-x., Yang, M. & Jia, X. (2023). MicroRNA expression profiles of stress susceptibility and resilience in the prelimbic and infralimbic cortex of rats after single prolonged stress. Frontiers in Psychiatry, 14. [Available online: https://www.frontiersin.org/journals/psychiatry/articles/10.3389/fpsyt.2023.1247714/full].
Hurley, D. (2015). Grandma’s Experiences Leave Epigenetic Mark on Your Genes. Discover Magazine. [Available online: https://www.discovermagazine.com/mind/grandmas-experiences-leave-epigenetic-mark-on-your-genes]. (Accessed: 17th July 2025).
Jawaid, A., Roszkowski, M. & Mansuy, I.M. (2018). Chapter Twelve – Transgenerational epigenetics of traumatic stress, Rutten, B.P.F. (ed.) Progress in Molecular Biology and Translational Science. Amsterdam: Academic Press, pp. 273-298. [Available online: https://www.sciencedirect.com/science/article/abs/pii/S187711731830053X#preview-section-introduction].
Jeon, H.J., Kang, E.S., Lee, E.H., Jeong, E.G., Jeon, J.R., Mischoulon, D. & Lee, D. (2012). Childhood trauma and platelet brain-derived neurotrophic factor (BDNF) after a three month follow-up in patients with major depressive disorder. Journal of Psychiatric Research, 46(7), pp. 966–972. [Available online: https://pubmed.ncbi.nlm.nih.gov/22551661/].
Jin, M.J., Jeon, H., Hyun, M.H. & Lee, S.H. (2019). Influence of childhood trauma and brain-derived neurotrophic factor val66met polymorphism on posttraumatic stress symptoms and cortical thickness. Scientific Reports, 9(1). [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6465240/].
Kalakoti, G., Vivek, A.T., Kamboj, A., Singh, A., Chakraborty, S. & Kumar, S. (2024). Comprehensive profiling of rRNA-derived small RNAs in Arabidopsis thaliana using rsRNAfinder pipeline. MethodsX, 12, p. 102494. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10711234/].
Kasinger, C., Schulz, AC., Ulke, C., Maercker, A., Beutel, M. & Brähler E. (2023). Historical and regional particularities in the prevalence of traumatic events and posttraumatic stress disorder in east and West Germany. BMC Public Health, 23(1). [Available online: https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-023-16534-6].
Klengel, T., Dias, B.G. & Ressler, K.J. (2015). Models of intergenerational and transgenerational transmission of risk for psychopathology in mice. Neuropsychopharmacology, 41(1), pp. 219–231. [Available online: https://www.nature.com/articles/npp2015249].
Labonté, B., Azoulay, N., Yerko, V., Turecki, G. & Brunet, A. (2014). Epigenetic modulation of glucocorticoid receptors in posttraumatic stress disorder. Translational Psychiatry, 4(3). [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3966043/].
Lacal, I. & Ventura, R. (2018). Epigenetic inheritance: Concepts, mechanisms and perspectives. Frontiers in Molecular Neuroscience, 11. [Available online: https://www.frontiersin.org/journals/molecular-neuroscience/articles/10.3389/fnmol.2018.00292/full].
Lawrence, S. & Scofield, R.H. (2024). Post traumatic stress disorder associated hypothalamic-pituitary-adrenal axis dysregulation and physical illness. Brain, Behavior, and Immunity – Health, 41, p. 100849. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC11401111/#sec4].
Lyons, D.M. & Parker, K.J. (2007). Stress inoculation‐induced indications of resilience in monkeys. Journal of Traumatic Stress, 20(4), pp. 423–433. [Available online: https://pubmed.ncbi.nlm.nih.gov/17721972/].
Maddox, S.A., Schafe, G.E. & Ressler, K.J. (2013). Exploring epigenetic regulation of fear memory and biomarkers associated with post-traumatic stress disorder. Frontiers in Psychiatry, 4, 62. [Available online: https://www.frontiersin.org/journals/psychiatry/articles/10.3389/fpsyt.2013.00062/full].
Matosin, N., Cruceanu, C. & Binder, E.B. (2017). Preclinical and clinical evidence of DNA methylation changes in response to trauma and chronic stress. Chronic Stress, 1. [Available online: https://journals.sagepub.com/doi/10.1177/2470547017710764].
Meaney, M.J. (2010). Epigenetics and the biological definition of gene × environment interactions. Child Development, 81(1), pp. 41–79. [Available online: https://pubmed.ncbi.nlm.nih.gov/20331654/].
Mehta, D., Pelzer, E.S., Bruenig, D., Lawford, B., McLeay, S., Morris, C.P., Gibson, J.N., Young, R.McD. & Voisey, J. (2019). DNA methylation from germline cells in veterans with PTSD. Journal of Psychiatric Research, 116, pp. 42–50. [Available online: https://www.sciencedirect.com/science/article/abs/pii/S002239561930233X].
Migicovsky, Z. & Kovalchuk, I. (2011). Epigenetic Memory in Mammals. Frontiers in Genetics, 2, 28. [Available online: https://www.frontiersin.org/journals/genetics/articles/10.3389/fgene.2011.00028/full].
Moore, D.S. (2015) The developing genome: An introduction to behavioral epigenetics. Oxford: Oxford University Press.
National Human Genome Research Institute (2025). Genetic imprinting. [Available online: https://www.genome.gov/genetics-glossary/Genetic-Imprinting] (Accessed: 17th July 2025).
National Public Radio (2021). For Many Who Were Present, The 9/11 Attacks Have Had A Lasting Mental Health Impact. [Available online: https://www.npr.org/sections/health-shots/2021/09/08/1035224815/for-many-there-that-day-the-attacks-on-9-11-have-had-lasting-mental-health-impac] (Accessed: 18th July 2025).
Neria, Y., Gross, R., Olfson, M., Gameroff, M.J., Wickramaratne, P., Das, A., Pilowsky, D., Feder, A., Blanco, C., Marshall, R.D., Lantigua, R., Shea, S. & Weissman, M.M. (2006). Posttraumatic stress disorder in primary care one year after the 9/11 attacks. General Hospital Psychiatry, 28(3), pp. 213–222. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3622521/#S6].
Nugent, N.R., Amstadter, A.B. & Koenen, K.C. (2008). Genetics of post‐traumatic stress disorder: Informing clinical conceptualizations and promoting future research. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 148C(2), pp. 127–132. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC2680188/].
Raise-Abdullahi, P., Meamar, M., Vafaei, A.A., Alizadeh, M., Dadkhah, M., Shafia, S., Ghalandari-Shamami, M., Naderian, R., Afshin Samaei, S. & Rashidy-Pour A. (2023). Hypothalamus and post-traumatic stress disorder: A Review. Brain Sciences, 13(7), p. 1010. [Available online: https://www.mdpi.com/2076-3425/13/7/1010].
Ridhuan, S.A., Caltabiano, A., Gillis, H., Giritlioğlu, A., Graff, A., Hampikian, L.E., Jones, A.K., Luetgerath, P., Pierce, A., Pomeroy, E. & Said-Mohamed, R. (2021). Advocating for a collaborative research approach on transgenerational transmission of trauma. Journal of Child and Adolescent Trauma, 14(4), pp. 527–531. [Available online: https://doi.org/10.1007/s40653-021-00369-7].
Rodgers, A.B., Morgan C.P., Bronson, S.L., Revello, S. & Bale, T.L. (2013). Paternal stress exposure alters sperm MicroRNA content and reprograms offspring HPA stress Axis Regulation. The Journal of Neuroscience, 33(21), pp. 9003–9012. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3712504/].
Rodgers, A.B. & Bale, T.L. (2015). Germ cell origins of posttraumatic stress disorder risk: The transgenerational impact of parental stress experience. Biological Psychiatry, 78(5), pp. 307–314. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4526334/].
Roth, T.L., Lubin, F.D., Funk, A.J. & Sweatt, J.D (2009). Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological Psychiatry, 65(9), pp. 760–769. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3056389/].
Sampedro-Piquero, P. & Begega, A. (2017). Environmental enrichment as a positive behavioral intervention across the lifespan. Current Neuropharmacology, 15(4), pp. 459–470. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC5543669/#sec6].
Sapolsky, R., Krey, L. & McEwen, B. (1985). Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. The Journal of Neuroscience, 5(5), pp. 1222–1227. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6565052/].
ScienceDirect (2024). Histone phosphorylation – an overview. [Available online: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/histone-phosphorylation] (Accessed: 21st July 2025).
SingleCare (2025). PTSD statistics 2025. [Available online: https://www.singlecare.com/blog/news/ptsd-statistics/] (Accessed: 20th July 2025).
Sinkkonen, L., Hugenschmidt, T., Berninger, P., Gaidatzis, D., Mohn, F., Artus-Revel, C.G., Zavolan, M., Svoboda, P. & Filipowicz, W. (2008). MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nature Structural & Molecular Biology, 15(3), pp. 259–267. [Available online: https://www.nature.com/articles/nsmb.1391].
Sirikantraporn, S. & Green, J. (2016). Introduction: Multicultural perspectives of intergenerational transmission of trauma. Journal of Aggression, Maltreatment & Trauma, 25(6), pp. 559–560. [Available online: https://www.tandfonline.com/doi/full/10.1080/10926771.2016.1194941].
Skvortsova, K., Iovino, N. & Bogdanović, O. (2018). Functions and mechanisms of epigenetic inheritance in animals. Nature Reviews Molecular Cell Biology, 19(12), pp.774–790. [Available online: https://www.nature.com/articles/s41580-018-0074-2].
Sun, Y., Luo Z-M., Guo, X-M., Su. D-F. & Liu, X. (2015). An updated role of microrna-124 in central nervous system disorders: A Review. Frontiers in Cellular Neuroscience, 9. [Available online: https://www.frontiersin.org/journals/cellular-neuroscience/articles/10.3389/fncel.2015.00193/full].
Sustainability Directory (2025). Intergenerational epigenetics. [Available online: https://pollution.sustainability-directory.com/term/intergenerational-epigenetics] (Accessed: 26th July 2025).
Švorcová, J. (2023). Transgenerational Epigenetic Inheritance of Traumatic Experience in Mammals. Genes, 14(1). [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9859285/].
Swales, D.A., Poggi Davis, E., Mahrer, N.E., Guardino, C.M., Shalowitz, M.U., Ramey, S.L. & Schetter, C.D. (2022). Preconception maternal posttraumatic stress and child negative affectivity: Prospectively evaluating the intergenerational impact of trauma. Development And Psychopathology, 35(2), pp. 619–629. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9309186/].
Tan, X., Luo, J., Ding, X. & Li, H. (2023). Preconception paternal mental disorders and child health: Mechanisms and interventions. Neuroscience & Biobehavioral Reviews, 144, p. 104976. [Available online: https://www.sciencedirect.com/science/article/pii/S0149763422004651].
Tanić, M. (2020). Chapter Twenty-Nine – Epigenome-wide association study (EWAS): Methods and applications, Tollefsbol, T. (ed.) Epigenetics Methods. London: Academic Press, 591-613. [Available online: https://www.researchgate.net/publication/342839824_Epigenome-wide_association_study_EWAS_Methods_and_applications].
University of Arkansas (2024). The Holocaust and Generational Trauma. [Available online: https://howdidtheholocaustaffect.uark.edu/2024/06/14/the-holocaust-and-generational-trauma/] (Accessed: 18th July 2025).
van Marle, H. (2015). PTSD as a memory disorder. European Journal of Psychotraumatology, 6(1). [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4393536/].
Vukojevic, V., Kolassa, I.T., Fastenrath, M., Gschwind, L., Spalek, K., Milnik, A., Heck, A., Vogler, C., Wilker, S., Demougin, P., Peter, F., Atucha, E., Stetak, A., Roozendaal, B., Elbert, T., Papassotiropoulos, A. & de Quervain, DJ. (2014). Epigenetic modification of the glucocorticoid receptor gene is linked to traumatic memory and post-traumatic stress disorder risk in genocide survivors. Journal of Neuroscience, 34(31), pp. 10274–10284. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6608273/].
Weaver, I. C., Cervoni, N., Champagne, F. A., D’Alessio, A. C., Sharma, S., Seckl, J. R., Dymov, S., Szyf, M., & Meaney, M. J. (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7(8), pp. 847–854. [Available online: https://pubmed.ncbi.nlm.nih.gov/15220929/].
Wicking, M., Steiger, F., Nees, F., Diener, S.J., Grimm, O., Ruttorf, M., Schad, L.R., Winkelmann, T., Wirtz, G. & Flor, H. (2016). Deficient fear extinction memory in posttraumatic stress disorder. Neurobiology of Learning and Memory, 136, pp. 116–126. [Available online: https://pubmed.ncbi.nlm.nih.gov/27686278/].
Wikipedia (2024). Epigenetics of PTSD. [Available online: https://en.wikipedia.org/wiki/Epigenetics_of_posttraumatic_stress_disorder] (Accessed: 17th July 2025).
Yehuda, R., Schmeidler, J., Wainberg, M., Binder-Bynes, K. & Duvdevani, T. (1998). Vulnerability to Posttraumatic Stress Disorder in Adult Offspring of Holocaust Survivors. American Journal of Psychiatry, 155(9), pp. 1163–1171. [Available online: https://psychiatryonline.org/doi/10.1176/ajp.155.9.1163].
Yehuda, R., Bell, A., Bierer, L.M. & Schmeidler, J. (2008). Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. Journal of Psychiatric Research, 42(13), pp. 1104–1111. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC2612639/].
Yehuda, R. & Bierer, L.M. (2009). The relevance of epigenetics to PTSD: Implications for the DSM‐V. Journal of Traumatic Stress, 22(5), pp. 427–434. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC2891396/].
Yehuda, R., Daskalakis, N.P., Lehrner, A., Desarnaud, F., Bader, H.N., Makotkine, I., Flory, J.D., Bierer, L.M. & Meaney, M.J. (2014). Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. American Journal of Psychiatry, 171(8), pp. 872–880. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4127390/#S15].
Yehuda, R., Daskalakis, N.P., Bierer, L. M., Bader, H.N., Klengel, T., Holsboer, F. & Binder, E.B. (2016). Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biological Psychiatry, 80(5), pp. 372–380. [Available online: https://www.biologicalpsychiatryjournal.com/article/s0006-3223%2815%2900652-6/fulltext].
Yehuda, R. & Lehrner, A. (2018). Intergenerational transmission of trauma effects: putative role of epigenetic mechanisms. World Psychiatry, 17(3), pp. 243-257. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6127768/].
Zaidan, H., Leshem, M. & Gaisler-Salomon, I. (2013). Prereproductive stress to female rats alters corticotropin releasing factor type 1 expression in ova and behavior and brain corticotropin releasing factor type 1 expression in offspring. Biological Psychiatry, 74(9), pp. 680–687. [Available online: https://www.biologicalpsychiatryjournal.com/article/S0006-3223(13)00361-2/abstract].
Zaidan, H., Galiani, D. & Gaisler-Salomon, I. (2021). Pre-reproductive stress in adolescent female rats alters oocyte microrna expression and offspring phenotypes: Pharmacological interventions and putative mechanisms. Translational Psychiatry, 11(1). [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7865076/].
Zain, J. (2012). Role of histone deacetylase inhibitors in the treatment of lymphomas and multiple myeloma. Hematology/Oncology Clinics of North America, 26(3), pp. 671–704. [Available online: https://www.sciencedirect.com/science/article/abs/pii/S088985881200007X].
Zhang, L., Liu, J. & Hou, Y. (2023). Classification, function, and advances in tsRNA in non-neoplastic diseases. Cell Death & disease, 14(11). [Available online: https://www.nature.com/articles/s41419-023-06250-9].
Zheng, X., Li, Z., Wang, G., Wang, H., Zhou, Y., Zhao, X., Cheng, CY., Qiao, Y. & Sun F. (2021). Sperm epigenetic alterations contribute to inter- and transgenerational effects of paternal exposure to long-term psychological stress via evading offspring embryonic reprogramming. Cell Discovery, 7(1). [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8553786/].
Zhu, H., Ding, G.L., Liu, X. & Huang, H. (2023). Developmental origins of diabetes mellitus: Environmental epigenomics and emerging patterns. Journal of Diabetes, 15(7), pp. 569–582. [Available online: https://www.researchgate.net/publication/370803095_Developmental_origins_of_diabetes_mellitus_Environmental_epigenomics_and_emerging_patterns].
Zhu, XB., Lu, JQ., Zhi, EL., Zhu, Y., Zou, SS., Zhu, ZJ., Zhang, F. & Li, Z. (2016). Association of a TDRD1 variant with spermatogenic failure susceptibility in the Han Chinese. Journal of Assisted Reproduction and Genetics, 33(8), pp. 1099–1104. [Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4974230/].
Zovkic, I.B., Meadows, J.P., Kaas, G.A. & Sweatt, J.D. (2013). Interindividual variability in stress susceptibility: A role for epigenetic mechanisms in PTSD. Frontiers in Psychiatry, 4. [Available online: https://www.frontiersin.org/journals/psychiatry/articles/10.3389/fpsyt.2013.00060/full#h7].
Zovkic, I.B. & Sweatt, J.D. (2013). Epigenetic Mechanisms in Learned Fear: Implications for PTSD. Neuropsychopharmacology, 38, pp. 77-93. [Available online: https://www.nature.com/articles/npp201279].




